Drug Trials Snapshots: XERMELO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to XERMELO Prescribing Information for complete information.
XERMELO (telotristat ethyl)
zer me’ loe
Lexicon Pharmaceuticals, Inc.
Approval date: February 28, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XERMELO is a drug used to treat diarrhea in adult patients with carcinoid syndrome. It is to be used in patients who did not reach adequate control of the diarrhea despite taking medicines for the carcinoid syndrome called somatostatin analog (SSA) therapy.
Carcinoid syndrome is a group of various signs and symptoms of a rare type of tumors called carcinoid tumors.
How is this drug used?
XERMELO is a tablet that is taken three times a day with food. XERMELO is to be taken in combination with SSA therapy.
What are the benefits of this drug?
XERMELO reduces bowel movement frequency.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trial. The average was based on the number of days with valid, non-missing data.
Table 2: Change from Baseline in Bowel Movements/Day Averaged Over 12 Weeks in Adult Patients with Carcinoid Syndrome Diarrhea
| Parameter | XERMELO | Placebo | |
|---|---|---|---|
| Bowel Movements/Day At Baselinea | Number of Patients | 45 | 45 |
| Baseline Mean (SD) Median (Min, Max) |
6.1 (2.1) 5.5 (3.5, 13.0) |
5.2 (1.4) 5.1 (3.5, 9.0) |
|
| Change From Baseline In Bowel Movements/Day Averaged Over 12 Weeks | Change Averaged over 12 Weeks: Mean (SD) Median (Min, Max) |
˗1.4 (1.4) -1.3 (-6.1, 1.6) |
˗0.6 (0.8) -0.6 (-2.7,0.8) |
| Estimate of Treatment Difference (97.5% CL)b | ˗0.8c (˗1.3, ˗0.3) |
--- |
CL=confidence limit; SD=standard deviation.
a Baseline Bowel Movements/Day was assessed over the 3-4 week screening/run-in period.
b Statistical tests used a blocked 2-sample Wilcoxon Rank Sum statistic (van Elteren test) stratified by the u5‑HIAA stratification at randomization. CLs were based on the Hodges-Lehmann estimator of the median paired difference.
c p
XERMELO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: XERMELO worked similarly in men and women.
- Race: The majority of patients in the clinical trial were white. The number of patients in other races was limited; therefore, differences in response to XERMELO among races could not be determined.
- Age: XERMELO worked similarly in patients below and above the age of 65.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex and age subgroups. Due to the small sample size, these exploratory analyses should be interpreted with caution.
Racial subgroup differences were not investigated since the majority of patients were white.
Table 3. Subgroup Analysis by Sex and Age: Change in Bowel Movement (counts/day) Averaged over the Double-Blind Treatment Period
| XERMELO N=45 | Placebo N=45 | |
|---|---|---|
| Men | N=21 | N=24 |
| Mean Change from Baseline (SD) | -1.21 (1.07) | -0.47 (0.83) |
| Hodges-Lehmann est. of treatment difference | -0.84 | -- |
| Women | N=24 | N=21 |
| Mean Change from Baseline (SD) | -1.63 (1.57) | -0.80 (0.81) |
| Hodges-Lehmann est. of treatment difference | -0.73 | -- |
| Age | N=26 | N=25 |
| Mean Change from Baseline (SD) | -1.32 (1.32) | -0.75 (0.79) |
| Hodges-Lehmann est. of treatment difference | -0.58 | -- |
| Age ≥ 65 | N=19 | N=20 |
| Mean Change from Baseline (SD) | -1.58 (1.44) | -0.46 (0.86) |
| Hodges-Lehmann est. of treatment difference | -1.04 | -- |
SD=standard deviation
Adapted from FDA Statistical review
What are the possible side effects?
XERMELO may cause serious constipation.
The most common side effects of XERMELO are nausea, headache, increased liver enzyme (called GGT), depression, gassiness, decreased appetite, leg swelling, and fever.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions, including laboratory abnormalities, in the clinical trials.
Table 4. Common Adverse Reactionsa by Treatment Group at 12-Weeks
| Adverse Reaction | XERMELO N=45 (%) | Placebo N=45 (%) |
|---|---|---|
| Nausea | 13 | 11 |
| Headache | 11 | 4 |
| Increased gamma-glutamyl-transferase (GGT) | 9 | 0 |
| Depressionb | 9 | 7 |
| Peripheral edema | 7 | 2 |
| Flatulence | 7 | 2 |
| Decreased appetite | 7 | 4 |
| Pyrexia | 7 | 4 |
a incidence of at least 5% in the XERMELO group and at an incidence greater than placebo
b including depression, depressed mood and decreased interest
XERMELO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in men and women
- Race: The majority patients in the clinical trial were white. The number of patients in other races was limited; therefore, differences in side effects among races could not be determined.
- Age: The risk of side effects was similar in patients below and above the age of 65.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events during the clinical trial by sex and age subgroup. Due to the small sample size, these exploratory analyses should be interpreted with caution.
Racial subgroups were not investigated since the majority of patients were white.
Table 6. Subgroup Analysis of Treatment-Emergent Adverse Events
| Demographic Subgroup | XERMELO N=45 x/n* (%) | Placebo N=45 x/n* (%) |
|---|---|---|
| Sex | ||
| Male | 17/21 (81) | 18/24 (75) |
| Female | 20/24 (83) | 21/21 (100) |
| Age Group | ||
| 65> | 22/26 (85) | 21/25 (84) |
| >= 65 years | 15/19 (79) | 18/20 (90) |
*x/n= number of patents with at least one treatment-emergent adverse event (x) in the subgroup (n)
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved XERMELO based on evidence from one clinical trial of 90 patients with diarrhea due to carcinoid syndrome. The trial was conducted in the USA, Canada, Germany, United Kingdom, Italy, France, Spain, Sweden, Belgium, Netherlands, Israel and Australia.
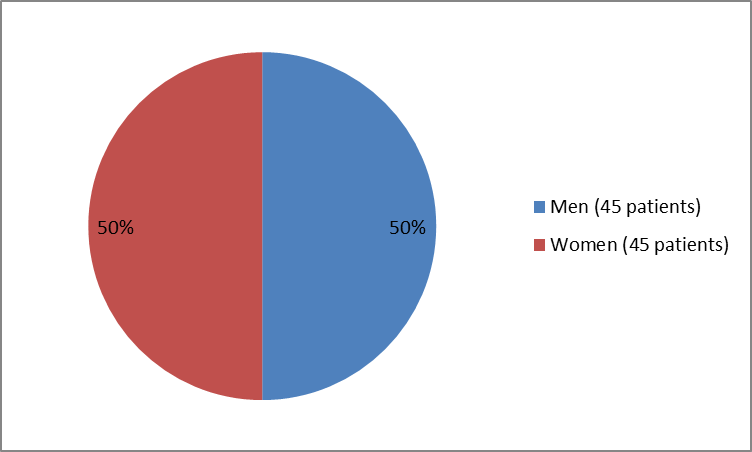
The figure below summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
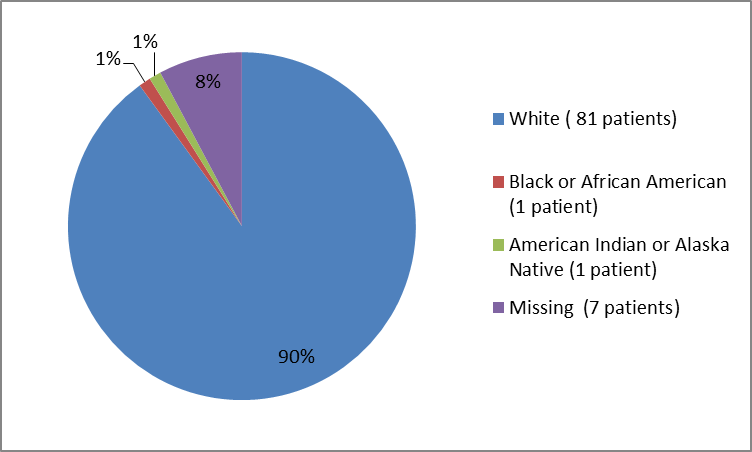
Figure 2 and Table 1 below summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 81 | 90 |
| Black or African American | 1 | 1 |
| American Indian or Alaska Native | 1 | 1 |
| Missing* | 7 | 8 |
* not reported
Clinical trial data
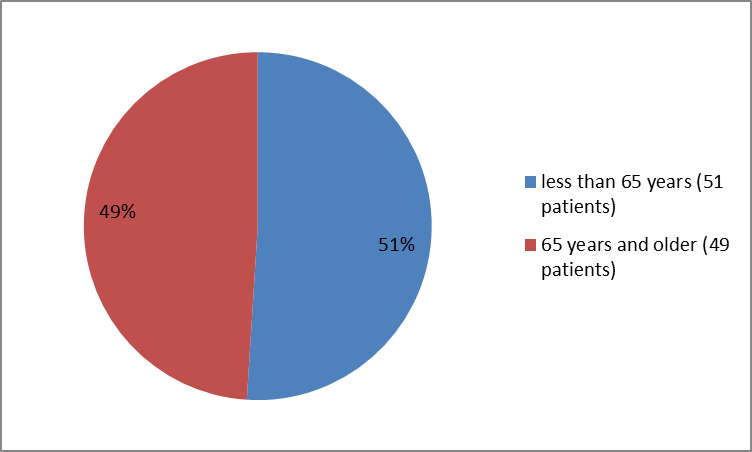
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trial.
Table 7. Baseline Demographics of Patients in the Clinical Trial
| Demographic Subgroup | XERMELO N=45 n (%) | Placebo N=45 n (%) | Total N=90 n (%) |
|---|---|---|---|
| Sex | |||
| Men | 21 (47) | 24 (53) | 45 (50) |
| Women | 24 (53) | 21 (47) | 45 (50) |
| Age Group | |||
| 65> | 26 (58) | 25 (56) | 51 (57) |
| ≥65 years | 19 (42) | 20 (44) | 39 (43) |
| Race | |||
| White | 41 (91) | 40 (89) | 81 (90) |
| Black or African American | 0 (0) | 1 (2) | 1 (1) |
| American Indian or Alaska Native | 0 | 1 (2) | 1 (1) |
| Missinga | 4 (9) | 3 (7) | 7 (8) |
| Ethnicity | |||
| Hispanic or Latino | 0 | 0 | 0 (0) |
| Not Hispanic or Latino | 45 (100) | 44 (98) | 89 (99) |
| Missinga | 0 | 1 (2) | 1 (1) |
a Race information not provided for 7 patients in France and ethnicity information was not provided for 1 of these patients
Clinical trial data
How were the trials designed?
The benefit and side effects of XERMELO were evaluated in one clinical trial of patients with carcinoid syndrome diarrhea despite the use of SSA therapy. Patients received either XERMELO or placebo three times daily for 12 weeks.
The benefit of XERMELO was evaluated by measuring the change from baseline in the number of daily bowel movements averaged over the 12-week treatment period.
How were the trials designed?
The safety and efficacy of XERMELO were established in a single, double-blind, placebo-controlled, randomized, multicenter trial of 12-week duration. All patients had a well-differentiated metastatic neuroendocrine tumor and carcinoid syndrome with 4 to 12 daily bowel movements despite the use of SSA therapy at a stable dose for at least 3 months.
The efficacy outcome measure was the change from baseline in the number of daily bowel movements averaged over the 12-week treatment period.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.