Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program
Drug Development Tools (DDTs) are methods, materials, or measures that have the potential to facilitate drug development. As described in the 21st Century Cures legislation, DDTs include biomarkers, clinical outcome assessments, and other methods, materials, or measures that aid drug development and regulatory review. To support DDT development efforts, FDA has established qualification programs for biomarkers, clinical outcome assessments, and for animal models for use under the Animal Rule.
The Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program is designed to expand DDT types by encouraging development of DDTs that are out of scope for existing DDT qualification programs but may still be beneficial for drug development.
Examples of submissions that might be considered for ISTAND include, but are not limited to:
Tools that may help enable remote or decentralized trials
- Application of patient-performed digital photography in dermatology trials
Tools that may advance our understanding of drugs
- Use of tissue chips (i.e., microphysiological systems) to assess safety or efficacy questions
- Development of novel nonclinical pharmacology/toxicology assays
Tools that leverage digital health technologies
- Use of artificial intelligence (AI)-based algorithms to evaluate patients, develop novel endpoints, or inform study design
- Use of novel digital health technologies (e.g., wearables) for patient assessment
Development programs needed for evaluation of adherence sensors
What outcomes are available under the ISTAND Pilot Program?
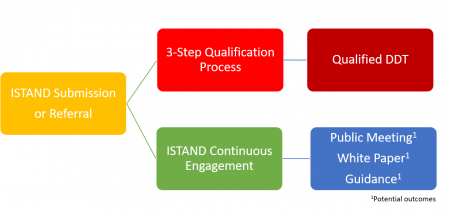
ISTAND’s goal is to support the development of novel approaches to drug development that may be acceptable for regulatory use. Some novel programs may be considered as DDTs eligible for qualification independent of a specific drug program and will go through the qualification process.
- A qualified DDT can be relied upon to have a specific interpretation and application in drug development and regulatory review within its stated context of use. Once qualified, DDTs will be available to use in any drug development program for the qualified context of use. Additionally, the qualified DDT can generally be included in IND, NDA, or BLA applications without needing FDA to reconsider and reconfirm its suitability.
Other programs may not optimally fit into the existing DDT qualification paradigms but still represent potential value to drug development. In such cases, ISTAND will work with the sponsor to find the best outcome, which may involve:
- a series of meetings with appropriate FDA staff to gain input to the sponsor,
- a public meeting to gain input on the novel approach,
- a “white paper” to raise considerations for implementation of the novel DDT,
- development of a guidance that states FDA’s position on how the novel tool might be used in drug development; or
- other means of FDA input and advice to support the DDT’s furtherance.
What information about submissions will be made public?
Submissions to the ISTAND Pilot Program that are admitted for review and deemed appropriate for qualification as an outcome are subject to the transparency provisions of the 21st Century Cures Act. More information on these provisions can be found on the Drug Development Tool Qualification Process: Transparency Provisions page. Submissions that do not proceed towards DDT qualification are not subject to those provisions.
What is the nature of the pilot?
In the pilot phase, FDA anticipates accepting 2-4 submissions into the ISTAND program each year with a triage and selection process that focuses on public health impact and feasibility of implementation.
For information on how to apply, see ISTAND Pilot Program Submission Process.