FDA Drug Safety Communication: FDA cautions about dosing errors when switching between different oral formulations of antifungal Noxafil (posaconazole); label changes approved
[ 1-04-2016 ]
The U.S. Food and Drug Administration (FDA) is cautioning that differences in dosing regimens between the two oral formulations of the antifungal Noxafil (posaconazole) have resulted in dosing errors. To help prevent additional medication errors, the drug labels were revised to indicate that the two oral formulations cannot be directly substituted for each other but require a change in dose. Direct mg for mg substitution of the two formulations can result in drug levels that are lower or higher than needed to effectively treat certain fungal infections.
Prescribers should specify the dosage form, strength, and frequency on all prescriptions they write for Noxafil. Pharmacists should request clarification from prescribers when the dosage form, strength, or frequency is not specified. Patients should talk to their health care professional before they switch from one oral formulation to the other.
Noxafil is approved in two oral formulations: an oral suspension and a delayed-release tablet. It is also approved as an intravenous solution for injection. Noxafil is used to help prevent certain invasive fungal infections caused by fungi called Aspergillus and Candida. Noxafil is used in patients who have an increased chance of getting these infections due to weakened immune systems. Noxafil oral suspension is also used to treat a fungal infection called thrush caused by Candida in the mouth or throat area.
Our review of the FDA Adverse Event Reporting System (FAERS) database identified cases of dosing errors with Noxafil. Noxafil was approved in 2006 as an oral suspension formulation. Since the approval of Noxafil delayed-release tablets in November 2013, FDA received eleven reports of the wrong oral formulations being prescribed and/or dispensed to patients. One case resulted in death, and an additional case resulted in hospitalization. According to the reports, these outcomes were a result of health care professionals not knowing that the two oral formulations cannot be substituted for each other without adjusting the dose due to differences in how the medicine is absorbed and handled by the body.
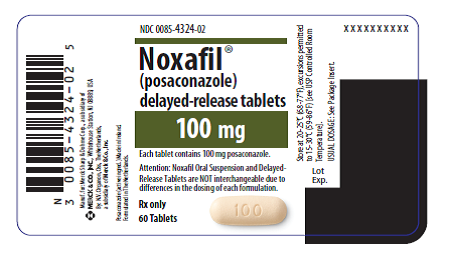
In addition to changes to the outer carton of Noxafil (see Photos), its manufacturer Merck revised the prescribing information and the patient information in the drug label to alert patients and their health care professionals that the two oral formulations of Noxafil cannot be substituted for each other.
We urge health care professionals and patients to report side effects involving Noxafil to the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of the page.
- Noxafil is used to prevent certain invasive Aspergillus and Candida fungal infections in patients 13 years or older, who have weakened immune systems.
- Noxafil oral suspension is also used to treat a Candida fungal infection of the mouth or throat called thrush.
- Noxafil is available in two oral formulations: delayed-release tablets and an oral suspension. The oral formulations are dosed differently, due to differences in how the medicine is absorbed and handled by the body.
- Noxafil is also available as an IV solution for injection.
- Common side effects can include diarrhea, nausea, fever, and low potassium levels in the blood.
- Due to reports of dosing errors when switching between the two oral formulations of Noxafil, the patient information and outer carton have been revised to indicate that the oral formulations cannot be substituted for each other due to differences in how each formulation is dosed.
- Take all of the medicine in your Noxafil prescription exactly as your health care professional tells you to take it.
- Seek medical attention right away if you:
- Develop severe diarrhea or vomiting.
- Notice swelling in an arm or leg, or experience shortness of breath.
- Notice a change in heart rate or heart rhythm.
- Other medicines may affect how Noxafil works. Tell your health care professional about all the medicines you take, including over-the-counter medicines and dietary supplements.
- Read the patient information leaflet you get along with your Noxafil prescription. It explains the risks associated with the use of Noxafil.
- Talk to your health care professional if you have questions or concerns about Noxafil.
- Report any side effects or medication errors from Noxafil to your health care professional and the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of this page.
- Due to reports of dosing errors when switching between the two oral formulations of Noxafil, the prescribing information and outer carton have been revised to indicate that the two oral formulations cannot be directly substituted for each other, due to differences in how each formulation is dosed.
- Prescribers should specify the dosage form, strength, and frequency on all prescriptions they write for Noxafil.
- Pharmacists should request clarification from prescribers when the dosage form, strength, or frequency is not specified.
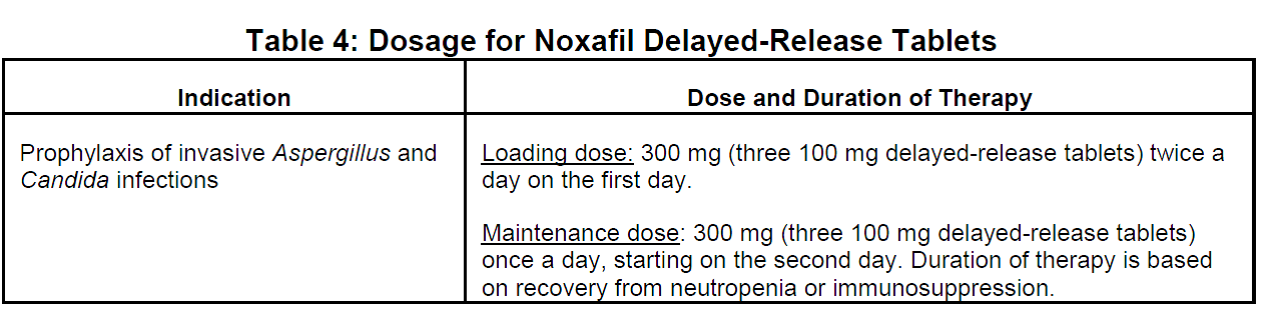
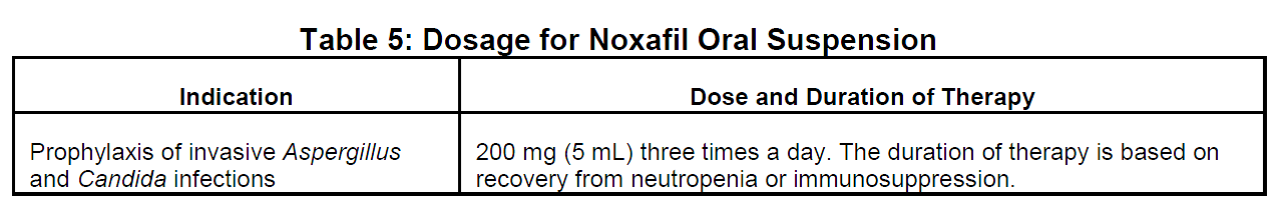
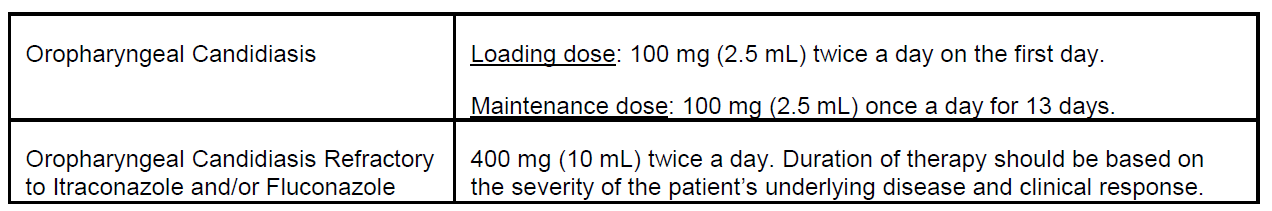
- Use caution when switching between Noxafil delayed-release tablets and Noxafil oral suspension, as the dosing is different for the two oral formulations. The delayed-release tablet has a higher bioavailability than the oral suspension. As a result, the dose and frequency of administration for Noxafil depend on the particular formulation used and the indication for use.
- Prescribers should follow the specific dosing instructions for each formulation. Incorrect dosage and administration can lead to subtherapeutic levels and potential for treatment failures, or higher levels and potential for adverse reactions.
- Advise patients to seek medical attention right away if they:
- Develop severe diarrhea or vomiting.
- Notice a change in heart rate or heart rhythm, or have a heart condition or circulatory disease. Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions.
- Notice swelling in an arm or leg, or experience shortness of breath.
- Have liver disease or develop itching, nausea or vomiting, their eyes or skin turn yellow, they feel more tired than usual or feel like they have the flu.
- Encourage patients to read the patient information leaflet they receive with their Noxafil prescription as there may be new information.
- Report adverse events or medication errors involving Noxafil to the FDA MedWatch program, using the information in the “Contact FDA” box at the bottom of this page.
Noxafil prescribing information:
Since the approval of the delayed-release tablet formulation of Noxafil (posaconazole) in November 2013, we have received eleven reports of the wrong oral formulation being prescribed and/or dispensed to patients. Noxafil is available in two oral formulations that cannot be directly substituted for one another due to differences in bioavailability. The delayed-release tablet has a higher bioavailability than the oral suspension. Thus, the dose and frequency of administration for Noxafil depend on the particular formulation used and the indication for use.
In one case report, a patient was taking Noxafil delayed-release tablets for prophylaxis of invasive Aspergillus and Candida infections, but the pharmacy replaced the tablets with the oral suspension directly without consideration of the different dose and frequency of administration of the oral suspension and resulted in an underdose. The patient was reported to have later died from a stroke related to an invasive Aspergillus infection.
The other ten case reports described patients switching from Noxafil oral suspension to the delayed-release tablets, but were prescribed or dispensed the same dose and frequency of administration as the oral suspension. For example, one patient received two 100 mg delayed-released tablets taken three times per day, which is twice the recommended Noxafil dose of three 100 mg delayed-release tablets taken once per day for prophylaxis of invasive Aspergillus and Candida infections. Some of the patients reported adverse reactions such as nausea and vomiting, and one patient presented to the hospital with these adverse reactions and was found to have a low serum potassium level.
The outer carton label changes to the oral suspension and delayed-release tablet formulations, approved November 2015, include the addition of an “Attention” statement:
Oral Suspension Label
Delayed-Release Tablet Label
Drug Safety Communication (PDF - 420KB)