Carton and Container Labeling Resources
For Industry
On this webpage
Who Is the Audience for This Website?
FDA’s carton and container labeling specific resources on this webpage are primarily directed to industry staff who develop carton and container labeling for prescription drugs.* For other prescription drug labeling resources such as those for FDA-approved patient labeling, carton and container labeling, biological products, generic drugs, and labeling databases, and product databases visit FDA’s Labeling Resources for Human Prescription Drugs. If you are a healthcare professional, patient, or caregiver, visit Frequently Asked Questions about Labeling for Prescription Medicines.
General Carton and Container Labeling Statutory/Regulatory Provisions
- Section 201(k) and (m) of FD&C Act: Statutory definition of “label” and “labeling”, respectively.
- 21 CFR 201.100(b): Prescription label requirements
- 21 CFR 201.10(i): Small label requirements
- 21 CFR 201.6: Misleading statement
- 21 CFR 201.15: Prominence of required label statements
- 21 CFR 610.60: Container label for biological products
- 21 CFR 610.61: Package labeling for biological products
- 21 CFR 610.62: Proper name; package label; legible type
- 21 CFR 610.63: Divided manufacturing responsibility to be shown
- 21 CFR 610.64: Name and address of distributor
Specific Container Label Statement Requirements
- 21 CFR 201.50: Statement of identity (established name)
- 21 CFR 201.51: Declaration of net quantity of contents
- 21 CFR 201.55: Statement of dosage (e.g., Recommended Dosage: see Prescribing Information)
- 21 CFR 201.10: Statement of ingredients (active and inactive)
- 21 CFR 201.17: Location of expiration date
- 21 CFR 201.18: Significance of control numbers (Lot number)
- 21 CFR 201.1: Name and place of business of manufacturer, packer, or distributor
- 21 CFR 201.2: National Drug Code (NDC)
- 21 CFR 201.15: Prominence of required label statements
- 21 CFR 201.20(a): FD&C Yellow No. 5
- 21 CFR 201.25: Bar code label requirements
Carton and Container Labeling Guidances and MAPPs
- Allowable Excess Volume and Labeled Vial Fill Size in Injectable Drug and Biological Products (final guidance)
- Bar Code Label Requirements Questions and Answers (final guidance)
- Child-Resistant Packaging Statements in Drug Product Labeling (final guidance)
- Gluten in Drug Products and Associated Labeling Recommendations (draft guidance)
- Harmonizing Compendial Standards With Drug Application Approval Using the USP Pending Monograph Process (draft guidance)
- Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting (final guidance)
- Liposome Drug Products: CMC; Human PK and Bioavailability; and Labeling Documentation (final guidance)
- Metered Dose Inhaler and Dry Powder Inhaler Drug Products - Quality Considerations (draft guidance)
- Naming of Drug Products Containing Salt Drug Substances (final guidance and MAPP)
- Product Identifiers Under the Drug Supply Chain Security Act Questions and Answers (final guidance) and Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance Policy (final guidance)
- Quality Attribute Considerations for Chewable Tablets (final guidance)
- Recommendations for Labeling Medical Products to Inform Users that the Product or Product Container is not Made with Natural Rubber Latex (final guidance)
- Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors (draft guidance)
- Safety Considerations for Product Design to Minimize Medication Errors (final guidance)
- Selection of the Appropriate Package Type Terms and Recommendations for Labeling Injectable Medical Products Packaged in Multiple-Dose, Single-Dose, and Single-Patient-Use Containers for Human Use (final guidance)
- Transdermal and Topical Delivery Systems - Product Development and Quality Considerations (draft guidance)
Other Carton and Container Labeling Resources
- Drug Product Nomenclature (2019 presentation and video)
- FDA List of Established Drug Names Recommended to Use Tall Man Lettering
- Improving Consistency of Information Between Carton-Container Labeling and Prescribing Information (2019 presentation and video)
- Institute for Safe Medication Practices’ List of Error-Prone Abbreviations, Symbols, and Dose Designations
- Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors (2019 presentation, and 2019 video)
- United States Pharmacopeia Resources (these resources require a USP subscription):
- Chapter <7> (Labeling) provides definitions and labeling standards for official USP articles including strength expression for injectable products, pharmacy bulk packages, imaging bulk packages, labeling for ferrules and cap overseals, aluminum use in parenteral nutrition, and expiration and beyond the use date.
- Chapter <659> (Packaging and Storage Requirements) provides definitions for packaging, package type terms for injectable medical products, noninjectable packaging containers, measuring devices (e.g., dosing cup, dosing spoon, medicine dropper, oral syringe), temperature, and storage
- Chapter <1091> (Labeling of Inactive Ingredients) provides guidelines for labeling of inactive ingredients to help promote consistency in labeling.
- Chapter <1121> (Nomenclature) provides general practices for drug product nomenclature.
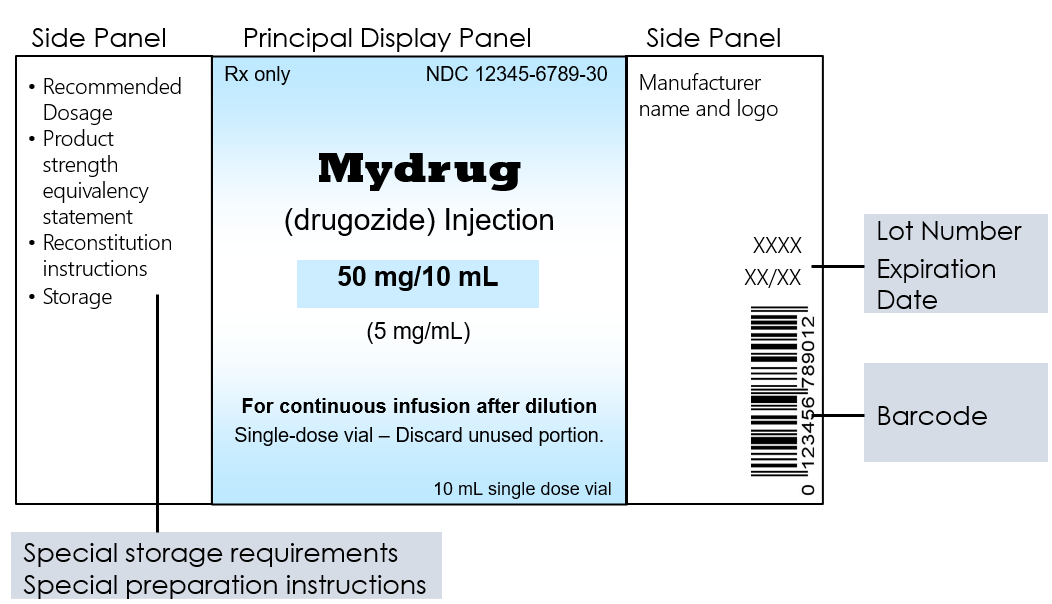
Example Container Label
Contact Information
For specific application or supplement questions or for general questions about prescription drug labeling, please visit Prescribing Drug Label Contact Information.