Effects of Realistic In Vitro Test Factors on the Aerosol Properties of Metered-Dose Inhalers
Background
Metered dose inhalers (MDIs) are drug-device combination products that use energy stored in a liquefied gas propellant, under pressure, for generating aerosols suitable for pulmonary drug delivery1. These products are mainstays in the treatment of asthma, COPD, and other respiratory diseases and CDER is engaged in wide ranging efforts to advance their development.
The size of droplets and particles emitted by metered dose inhalers (MDI) that pass through the mouth-throat (MT) region play a key role in determining how inhaled particles are deposited in the respiratory tract. Understanding the effects of in vitro test variables on the particle size distribution of aerosols exiting an anatomical MT model may therefore help improve the predictability of in vivo lung disposition and the development of generic and branded drug inhalers.
In a recent study, CDER’s Office of Generic Drugs investigated how the aerodynamic particle size distribution (APSD) and droplet size distribution of commercial solution and suspension MDIs are affected by in vitro testing conditions. Five factors were examined including different geometry and sized MT models, inhalation profiles representing strong, medium, and weak inhalation, two commonly used MT model coatings, MDI insertion angles, and two MDI firing points after the start of inhalation profiles. Two analytical methods, cascade impactor and laser diffraction (LD), were used to measure APSD and droplet size distributions, respectively.
The regulatory science challenge
The procedures and instrumentation for determining the APSD are quite complex with many factors that can vary in test design. One such complex factor is the MT model. These anatomical models are used to provide a more realistic assessment of MT deposition than a simple induction port, and they allow for estimation of a range of depositions representing population variance in lieu of a clinical study, but there are many models an investigator can choose from (Figure 1). The choice of the MT model and inhalation profiles, along with other experimental factors (coating type, insertion angles of the MDI, etc.) is usually made by each individual laboratory. The impact of the various choices on the APSD of MDI products has not been systematically assessed.
The most common technique for assessing size distribution of an MDI aerosol is the cascade impactor as it provides a quantitative link between mass of the drug deposited and its aerodynamic particle size2. In contrast to the cascade impactor-based measurements, LD provides assessment of the droplet size distribution of the entire aerosol plume over the duration of the spray. Measurement of droplet size distribution using LD is included as part of the recommended in vitro studies for establishing bioequivalence (BE) found in FDA product-specific guidances (PSGs) for nasal sprays, but not for MDI products, and whether the LD based droplet size distribution of aerosols exiting MT models are relevant for MDI product performance has not been established.
The study
This study undertook a systematic analysis of the effects of the five different factors with a goal of better understanding the effects of the experimental factors on APSD and droplet size distribution of the MDI’s in a realistic in vitro set-up. In the testing, inhalers were actuated to deliver the drug substance through a simple induction port or an MT model to a cascade impactor to separate aerosolized particles of different densities and dynamic shape. MDI products used included two suspension MDIs, Flovent® HFA (fluticasone propionate, 0.22 MG/INH) and Symbicort® (budesonide; formoterol fumarate, 0.16 MG/INH; 0.0045 MG/INH), and a model solution MDI, Atrovent® HFA (ipratropium bromide, 0.021 MG/INH). In the study, a total of five factors and 10 different MT models were studied (Figure 1).
Figure 1: Mouth throat (MT) models used in the study. AIT: Alberta Idealized Throat; OPC: Oropharyngeal Consortium; USP: United States Pharmacopoeia; VCU: Virginia Commonwealth University.
Systematically analyzing factors that influence aerosol particle size distribution
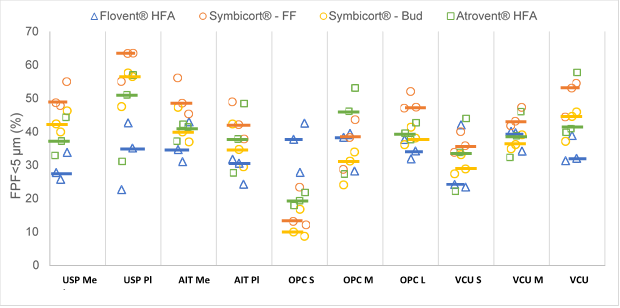
In this systematic analysis of the five in vitro factors on the particle size distribution of the two model suspension MDIs, the MT geometries appeared to have the strongest effects on APSD-derived parameters, while the effects of inhalation profiles depended on the product type. The choice of the MT model significantly affected the fine particle fraction (FPF) <5 µm (Figure 2), mass median aerodynamic diameter and in vitro lung dose (dose exiting the MT model) for all the three MDI products. Furthermore, the effects of the investigated factors on the APSD and droplet size distribution were often product-specific and unrelated to the formulation type (i.e., suspension or solution).
Figure 2: Fine Particle Fraction<5 μm (FPF<5 μm) of Flovent® HFA, Symbicort® (Formoterol Fumarate Dihydrate (FF) and Budesonide (Bud)) and Atrovent® HFA for the different MT models. Individual data point: mean (N=3) for a given test condition. Horizontal line represents the median. FF: formoterol fumarate dihydrate, Bud: budesonide, Me: metal, Pl: plastic, S: small, M: medium and L: large.
Results and conclusions
Based on the results, the researchers in this study3 concluded that during the development of inhalers, applicants who utilize more realistic in vitro studies should consider how the experimental conditions, particularly the MT model type and inhalation profiles, affect the amount and particle size distribution of aerosols exiting an anatomical MT model when designing their studies. MT geometries appear to have the strongest effects on APSD-derived parameters, while the effects of inhalation profile depended on the product type. LD may serve as an additional supporting characterization method rather than an alternative to cascade impactor-based realistic in vitro methods for the estimation of the particle size distribution of MDIs, given the limited and product-specific correlations that were found between the APSD-derived parameters and LD measurements.
How does this research improve generic drug development and support their approval?
Development of generic versions of inhalation products is challenging because the generic product should generally have the same delivery of small aerosol particles through the mouth and throat and into the lungs as the brand product. CDER scientists are helping develop more realistic laboratory models of the mouth-throat (MT) region that allow the generic industry to efficiently test their products and speed access to generic products. The experiments in this study measure the effects of in vitro test variables on the particle size distribution of aerosols exiting an anatomical MT model and may improve the predictability of in vivo lung disposition which could accelerate the development of generic as well as branded drug inhalers.
(This Impact Story is based on the Aerosol Society DDL Conference Paper by Sneha Dhapare, Abhinav Ram Mohan, and Bryan Newman - Office of Research and Standards, Office of Generic Drugs, Center for Drug Evaluation and Research, Food and Drug Administration3)
References, select publications
- See FDA draft guidance Metered Dose Inhaler (MDI) and Dry PowderInhaler (DPI) Products – Quality Considerations for a complete definition of these products.
- Bonam, M., Christopher, D., Cipolla, D., Donovan, B., Goodwin, D., Holmes, S., ... & Wyka, B. (2008). Minimizing variability of cascade impaction measurements in inhalers and nebulizers. AAPS Pharmscitech, 9(2), 404-413.
- Dhapare S, Newman B, Svensson M, Elfman P, Sandell D, Winner L, Bulitta J, Hochhaus G: Factors Influencing Plume Characteristics of Metered Dose Inhalers (MDIs) Following Passage through Bio- relevant Mouth-Throat Model. (Abstract). Presented at: Respiratory Drug Delivery 2021, Virtual Conference. May 4-7, 2021; 1; 301-306; (online).