Drug Trial Snapshot: RECARBRIO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.r text
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the RECARBRIO Package Insert for complete information.
RECARBRIO (imipenem, cilastatin, and relebactam)
reh-CAR-bree-oh
Merck Sharp & Dohme Corp.
Approval date: July 16, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RECARBRIO is a drug for adults who do not have additional options to treat:
- Complicated urinary tract infection (cUTI) including infection of the kidneys (pyelonephritis) caused by specific bacteria or,
- Complicated intra-abdominal infections (cIAI) (serious infections that extend into the intra-abdominal cavity and require treatment in the hospital) caused by specific bacteria.
RECARBRIO is a combination of the previously approved drugs (imipenem/cilastatin and a new drug (relebactam). RECARBRIO should only be used when the infection is caused by bacteria or strongly suspected to be caused by bacteria.
How is this drug used?
RECARBRIO is a drug administered by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV, infusion. It takes about 30 minutes to receive a RECARBRIO infusion.
RECARBRIO is given every 6 hours for 4 to 14 days.
What are the benefits of this drug?
In patients who have few or no other treatment options, RECARBRIO treats cIAI and cUTI.
What are the benefits of this drug (results of trials used to assess efficacy)?
The determination of efficacy of RECARBRIO was supported in part by the previous findings of the efficacy of imipenem/cilastatin for the treatment of cIAI and cUTI. The contribution of relebactam to RECARBRIO was established in vitro and in animal models of infection. RECARBRIO was studied in two, randomized, blinded, active-controlled, trials in adults with cIAI and cUTI. The trials were designed primarily for safety and provided limited efficacy information.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trials that evaluated RECARBRIO provided limited benefit information. Therefore, differences in how the drug worked among sex, race, and age subgroups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The trials that evaluated RECARBRIO provided limited efficacy information to determine if there were differences in how well the drug worked in sex, race, and age subgroups.
What are the possible side effects?
RECARBRIO may cause serious side effects such as severe allergic reactions, seizures, and antibiotic-associated diarrhea called clostridium difficile.
The most common side effects of RECARBRIO are diarrhea, nausea, headache, vomiting and abnormal liver tests.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients from both trials.
Table 2. Adverse Reactions Occurring in Greater Than or Equal to 1% of Patients in Trials 1 and 2
|
Disorders |
|
RECARBRIOa |
Imipenem/Cilastatin plus placebob |
|
Blood and lymphatic system disorders |
Anemiac |
2 (1%) |
4 (2%) |
|
Gastrointestinal disorders |
Diarrhea |
12 (6%) |
9 (4%) |
|
|
Nausea |
12 (6%) |
12 (6%) |
|
|
Vomiting |
7 (3%) |
4 (2%) |
|
General disorders and administration site conditions |
Phlebitis/Infusion site reactionsd |
5 (2%) |
3 (1%) |
|
|
Pyrexia |
5 (2%) |
3 (1%) |
|
Laboratory Investigations |
Alanine aminotransferase increased |
7 (3%) |
4 (2%) |
|
|
Aspartate aminotransferase increased |
6 (3%) |
3 (1%) |
|
|
Lipase increased |
3 (1%) |
4 (2%) |
|
|
Blood creatinine increased |
1 (<1%) |
3 (1%) |
|
Nervous system disorders |
Headache |
9 (4%) |
5 (2%) |
|
|
Central nervous system adverse reactionse |
2 (1%) |
5 (2%) |
|
Vascular disorders |
Hypertensionf |
4 (2%) |
6 (3%) |
b Imipenem/Cilastatin (500mg/500mg) + Placebo, IV every 6 hours.
c Anemia includes anemia and hemoglobin decreased.
d Infusion site reactions include infusion site phlebitis, infusion site erythema and infusion site pain.
e Central nervous system adverse reactions include agitation, apathy, confusional states, delirium, disorientation, slow speech, and somnolence
f Hypertension includes hypertension and blood pressure increased.
RECARBRIO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Therefore, differences in the occurrence of side effects could not be determined.
- Age: The occurrence of side effects was similar among patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the most common adverse reactions, diarrhea and nausea, by sex and age subgroup. Race subgroups were not tested because of the population was predominantly White.
Table 3. Adverse Events Reported in ≥ 1% of Subjects in Trial 1 and Trial 2 by Treatment Group and Sex
|
Adverse Event |
RECARBRIOa |
Imipenem/Cilastatin plus placebob |
||
|
Men |
Women |
Women |
Men |
|
|
Diarrhea |
8 (3.7%) |
4 (1.8%) |
5 (2.3%) |
4 (1.9%) |
|
Nausea |
5 (2.3%) |
7 (3.2%) |
6 (2.8%) |
6 (2.8%) |
aImipenem/Cilastatin (500mg/500mg) + Relebactam (250mg), IV every 6 hours.
bImipenem/Cilastatin (500mg/500mg) + Placebo, IV every 6 hours.FDA Review
Table 4. Adverse Events Reported in ≥ 1% of Subjects in Trial 1 and Trial 2 by Treatment Group and Age Group
|
Adverse Event |
RECARBRIOa |
Imipenem/Cilastatin plus |
||
|
< 65 years |
> 65 years |
< 65 years |
> 65 years |
|
|
Diarrhea |
6 (2.7%) |
6 (2.7%) |
5 (2.3%) |
4 (1.9%) |
|
Nausea |
10 (4.6%) |
2 (0.9%) |
11 (5.1%) |
1 (0.5%) |
aImipenem/Cilastatin (500mg/500mg) + Relebactam (250mg), IV every 6 hours.
bImipenem/Cilastatin (500mg/500mg) + Placebo, IV every 6 hours.
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
RECARBRIO was studied in two clinical trials (Trial 1/NCT01505634, Trial 2/NCT01506271) of 430 patients with cUTI or cIAI. The trials were conducted in Europe, South America, United Sates, Asia Pacific, Africa, and Mexico.
The FDA primarily considered previous findings of the efficacy and safety of imipenem/cilastatin in the treatment of cIAI and cUTI as well as the evidence from the laboratory and animal studies showing the contribution of relebactam.
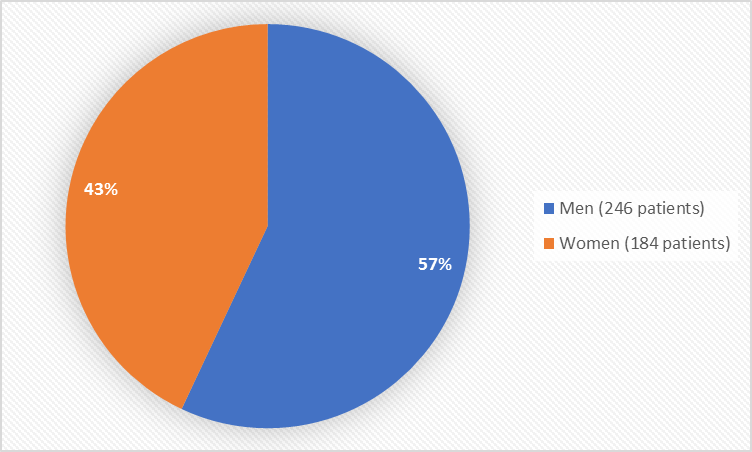
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
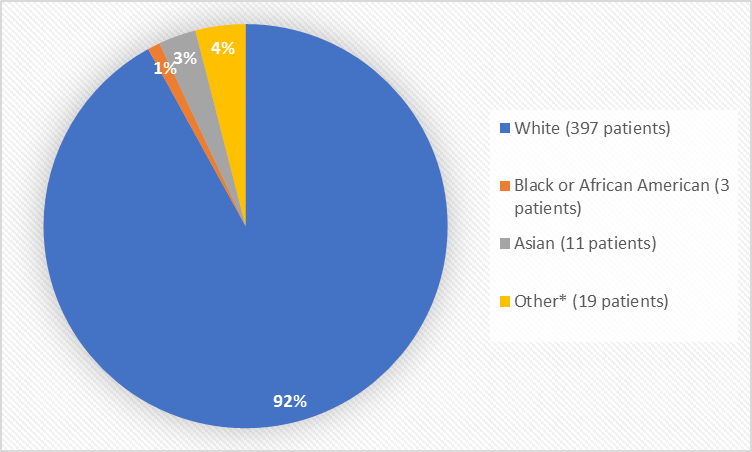
Figure 2. Baseline Demographics by Race (safety population)
*Other includes American Indian, or Alaska Native
FDA Review
Table 1. Demographics of Trials by Race (safety population)
|
Race |
Number of Patients |
Percentage of Patients |
|
White |
397 |
92% |
|
Black or African American |
3 |
1% |
|
Asian |
11 |
3% |
|
American Indian or Alaska Native |
2 |
Less than 1 |
|
Other |
17 |
4% |
FDA Review
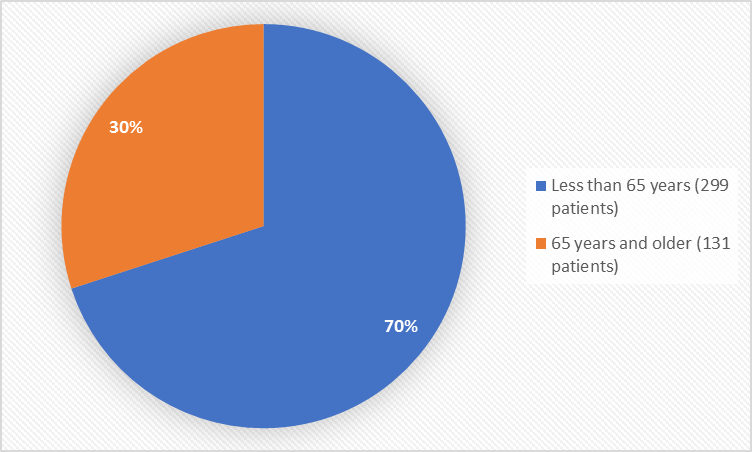
Figure 3 summarizes the percentage of patients by age group in the clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Who participated in the trials?
The table below summarizes demographics of patients in Trial 1 and Trial 2.
Table 5. Demographic Characteristics (safety population)
|
Demographic Parameters |
|
RECARBRIOa |
Imipenem/Cilastatin plus placebo |
Total |
|
Sex |
Men |
124 (57.4%) |
122 (57.0%) |
246 (57.2%) |
|
|
Women |
92 (42.6%) |
92 (43.0%) |
184 (42.8%) |
|
Race |
White |
198 (91.7%) |
199 (93.0%) |
397 (92.3%) |
|
|
Black or African American |
2 (0.9%) |
1 (0.5%) |
3 (0.7%) |
|
|
Asian |
4 (1.9%) |
7 (3.3%) |
11 (2.6%) |
|
|
American Indian or Alaska Native |
2 (0.9%) |
0 (0.0%) |
2 (0.5%) |
|
|
Other |
10 (4.6%) |
7 (3.3%) |
17 (3.9%) |
|
Age |
Mean years (SD) |
52.5 (18.7) |
52.0 (18.7) |
52.2 (18.7) |
|
|
Median (years) |
55 |
54 |
55 |
|
|
Min, max (years) |
18, 90 |
18, 88 |
18, 90 |
|
Age Group |
< 65 years |
149 (69.0%) |
150 (70.1%) |
299 (69.5%) |
|
|
≥ 65 years |
67 (31.0%) |
64 (29.9%) |
131 (30.5%) |
|
Ethnicity |
Hispanic or Latino |
15 (6.9%) |
9 (4.2%) |
24 (5.6%) |
|
|
Not Hispanic or Latino |
191 (88.4%) |
192 (89.7%) |
383 (89.1%) |
|
|
Not reported/unknown |
10 (4.6%) |
13 (6.1%) |
23 (5.3%) |
|
Region |
Africa |
4 (1.9%) |
2 (0.9%) |
6 (1.4%) |
|
|
Asia Pacific |
4 (1.9%) |
5 (2.3%) |
9 (2.1%) |
|
|
Europe |
184 (85.2%) |
190 (88.8%) |
374 (87.0%) |
|
|
Mexico |
0 |
1 (0.5%) |
1 (0.2%) |
|
|
South America |
15 (6.9%) |
8 (3.7%) |
23 (5.3%) |
|
|
United States |
9 (4.2%) |
8 (3.7%) |
17 (4.0%) |
aImipenem/Cilastatin (500mg/500mg) + Relebactam (250mg), IV every 6 hours.
FDA Review
How were the trials designed?
Trial 1 enrolled adult patients hospitalized with cUTI. Trial 2 enrolled adult patients hospitalized with cIAI that required surgery or drainage. In both trials, patients were assigned to either imipenem/cilastatin with varying doses of relebactam or imipenem/cilastatin with placebo intravenously, every 6 hours for 4 to 14 days. Neither the patients nor the investigators knew which treatment was being given until after the trial was completed.
The trials provided data primarily for evaluation of side effects.
How were the trials designed?
There were two randomized, active-control trials in adult patients. Trial 1 enrolled hospitalized patients with cUTI. Trial 2 enrolled adult patients hospitalized with cIAI that required surgery or drainage. In both trials, patients were randomly assigned to either imipenem /cilastatin with varying doses of relebactam, or imipenem/cilastatin with placebo intravenously every 6 hours for 4 to 14 days.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.