Zika Virus Diagnostic Development
Types of Diagnostic Tests | Available Diagnostics | Support for Diagnostic Development | Zika Virus Reference Materials | LDTs & Zika Virus | Contact FDA | Translations (Spanish, Portuguese)
FDA encourages commercial diagnostic developers and researchers developing laboratory developed tests (LDTs) for Zika virus to submit an Emergency Use Authorization (EUA) request or consider pursuing a premarket submission. FDA will work interactively with developers to support such requests.
FDA has rapidly granted Emergency Use Authorizations for several in vitro diagnostic (IVD) devices, and on May 23, 2019 authorized marketing of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. The ZIKV Detect is the first Zika diagnostic test the FDA has allowed to be marketed in the U.S. See additional information under Available Diagnostics below.
Types of Diagnostic Tests
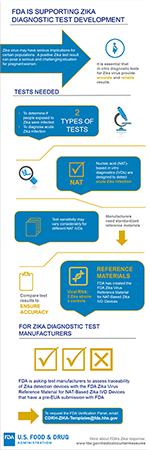
Two types of diagnostic tests are needed for Zika virus: (1) tests to diagnose acute infection; and (2) tests to assess whether individuals, especially pregnant women, who were potentially exposed to Zika virus were actually infected. More: Testing for Zika Virus, from CDC
Available Diagnostics
For a list of available diagnostics, see Medical Products on the Zika Virus Response Updates from FDA page.
On May 23, 2019, FDA authorized marketing (PDF, 175 KB) of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is the first Zika diagnostic test the FDA has allowed to be marketed in the U.S. FDA reviewed the data for the test through the De Novo premarket review pathway. Previously, tests for detecting Zika virus immunoglobulin (IgM) antibodies—including the ZIKV Detect 2.0 IgM Capture ELISA—had been authorized only for emergency use under the FDA’s Emergency Use Authorization (EUA) authority. For more information, see Serological assays on the Zika Virus Response Updates from FDA page
The ZIKV Detect 2.0 IgM Capture ELISA marketing authorization does not impact the availability of the nucleic acid testing-based assays available under EUA to diagnose active Zika infection. See Emergency Use Authorization (EUA) for information about Zika virus diagnostics available under EUA.
Laboratory personnel using Zika diagnostic assays under EUA are encouraged to report performance concerns directly to FDA at [email protected], in addition to reporting concerns to the manufacturer.
Performance characteristics of Zika virus diagnostic tests
FDA has posted new tables detailing performance characteristics of Zika virus diagnostic tests (assays) currently available for use under EUA. The tables include information about analytical sensitivity, along with other performance characteristics determined during EUA evaluation. (May 3, 2018)
Support for Diagnostic Test Development
FDA is working interactively with Zika virus diagnostic developers to help accelerate development programs and requests for EUAs. Draft EUA review templates delineating data requirements for a Zika virus diagnostic EUA are available by sending a request to: [email protected].
Zika Virus Reference Materials
Publication
FDA Zika virus reference panel for molecular-based diagnostic devices supports product testing for Emergency Use Authorization and 510(k) submissions (November 2019) - read the full publication in The Journal of Molecular Diagnostics
In this section:
- Reference materials for NAT-based IVD devices
- International reference preparation
- Reference materials for serological tests
 Reference materials for NAT-based IVD devices
Reference materials for NAT-based IVD devices
There are two primary blood diagnostic tests: nucleic acid tests that identify infection by confirming the presence of a virus’ genetic material (RNA) and serological tests that identify proteins (antibodies) produced by the body's immune system when it detects harmful organisms, such as Zika virus, in the blood. Nucleic acid (NAT)-based IVD devices for viral RNA are the most sensitive method available to detect acute (current) Zika virus infection. However, the sensitivity of NAT-based methods may vary considerably across assays, and standardized reference materials are needed to facilitate product development. One of the conditions of authorization for a ZIKV NAT-based IVD device under an EUA, is for the EUA holder to assess traceability of their device with an FDA-recommended reference material. Traceability refers to tracing analytical sensitivity/reactivity back to a FDA recommended reference material.
To support fulfillment of an EUA condition of authorization to assess traceability, FDA has created the FDA Zika Virus Reference Materials for NAT-based IVD devices, which contains RNA from two current Zika virus strains in human plasma and three controls for blind testing. The FDA Zika Virus Reference Materials are available upon request to Zika device developers who have a pre-EUA submission with the agency and have established the analytical and clinical performance of their assay. FDA will consider requests for material to be used solely for research purposes on a case-by-case basis. The reference material is made available without cost.
To request the FDA Zika Virus Reference Materials for NAT-based IVD devices, email [email protected].
As a courtesy only, FDA is providing a list of potential sources of Zika-positive controls and/or verification/validation panels:
- Heat-inactivated virus in plasma available from European Virus Archive goes Global (EVAg)
- Freeze-dried, quantified virus available from European Virus Archive goes Global (EVAg)
- Purified, quantified virus RNA available from Vircell
- Quantified recombinant virus in human plasma available from SeraCare Life Sciences
Three of the above entities are in Europe, so there may be some steps required to bring materials into the United States. For information on importing reference biological material into the U.S. for use with the Zika NAT-based IVD devices, please visit the following link or contact [email protected].
International reference preparation
The World Health Organization (WHO) provides International Biological Reference Preparations which serve as reference sources of defined biological activity expressed in an internationally agreed unit. The WHO International Standard for Zika virus RNA for use in NAT-Based assays is available from WHO Collaborating Center Paul-Ehrlich Institut (PEI). Also see: Collaborative Study to Evaluate a Candidate WHO International Standard for Zika Virus for NAT-Based Assays (PDF, 700 KB)
Reference materials for serological tests
In July 2017, FDA also made available a panel of human plasma samples to aid in the regulatory evaluation of serological tests to detect recent Zika virus infection. Serological tests are especially important because there is often a small window when the virus’ genetic material is detectable. However, development of these types of tests has been particularly challenging because antibodies produced by the body to fight Zika virus are difficult to differentiate from antibodies produced to fight related viruses, such as dengue and West Nile viruses.
The FDA’s sample panel consists of plasma samples from anonymous individuals infected with Zika, West Nile, or dengue viruses. Although the panel is not for research purposes, diagnostic developers can use these samples to assess whether their tests can help distinguish recent Zika virus infection from infection with West Nile or dengue viruses. Using the same serological panel to evaluate different devices available under Emergency Use Authorization (EUA) will help public health professionals compare the performance of different Zika virus tests.
The FDA panel is available to developers who have interacted with the FDA through the pre-EUA process and have devices that are in the final stages of validation. As of December 12, 2018, the FDA had granted EUAs to five serological tests for detection of recent Zika virus infection. On May 23, 2019, FDA authorized marketing of the ZIKV Detect 2.0 IgM Capture ELISA to detect Zika virus immunoglobulin (IgM) antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is the first Zika diagnostic test the FDA has allowed to be marketed in the U.S. Previously, tests for detecting Zika virus immunoglobulin (IgM) antibodies—including the ZIKV Detect 2.0 IgM Capture ELISA—had been authorized only for emergency use under the FDA’s EUA authority. Developers planning a future premarket submission will have priority to receive the panel of human plasma samples, considering the grant of a De Novo classification request for the ZIKV Detect 2.0 IgM Capture ELISA on May 23, 2019.
Developers interested in requesting a panel may contact the agency at [email protected].
The panel was prepared using samples from Zika virus-infected individuals provided by Blood Systems Research Institute (BSRI) from a study supported by Contract No. HHSN268201100001I from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH). The content of this press release is solely the FDA’s responsibility and does not necessarily represent the official views of BSRI, the NHLBI, or the NIH. The samples from individuals infected with dengue and West Nile virus were obtained separately by the FDA.
LDTs and Zika Virus
Laboratory developed tests are a subset of in vitro diagnostic devices that are intended for clinical use and designed, manufactured, and used within a single laboratory. Historically, FDA has generally not enforced premarket review and other applicable FDA requirements for LDTs because such tests were relatively simple lab tests and generally available on a limited basis. However, due to advances in technology and changes in business models, LDTs have evolved and proliferated significantly since the FDA first obtained comprehensive authority to regulate all in vitro diagnostics as devices in 1976. Some LDTs are now more complex, have a nation-wide reach and present higher risk.
Patients, as well as their physicians, depend on FDA to assure the tests they use to make medical decisions are accurate, reliable, and clinically meaningful. Recently, several developers announced they would be developing and making LDTs for Zika virus available to patients.
Zika virus may have serious implications for certain populations. For example, given the potential association of microcephaly and other poor pregnancy outcomes and Zika virus, a positive Zika test results poses a serious and challenging situation for pregnant women. Thus it is essential that in vitro diagnostic tests for Zika virus provide accurate and reliable results.
As such, FDA has requested developers of LDTs for Zika virus to submit information about their tests to help FDA better understand their design, validation, and performance characteristics. While FDA recognizes the need for expanding laboratory testing capacity for Zika virus, and encourages laboratories to develop Zika in vitro diagnostic tests, these tests should not be used for clinical diagnoses without FDA’s approval, clearance, or authorization. FDA is encouraging developers of LDTs for Zika virus to submit a request for an EUA; FDA will work interactively with LDT developers to support such requests.
Contact FDA
Diagnostic Product Sponsors/Manufacturers
Draft EUA review templates for Zika, and Zika virus reference materials are available by email request to:
[email protected]
Laboratories
Laboratory personnel using Zika diagnostic assays under EUA are encouraged to report performance concerns directly to FDA at [email protected], in addition to reporting concerns to the manufacturer.
For questions regarding importing reference biological material into the U.S. for use with the Zika NAT-based IVD devices, contact [email protected].
Translations
Note: Spanish and Portuguese translations of this page are archived, and were last updated on the date listed at the bottom of the archived page.
Related Links
- Zika Virus EUA Information (Emergency Use Authorization)
- FDA is Supporting Zika Diagnostic Test Development - Infographic (PDF, 120 KB)
- Zika Virus Response Updates from FDA
- In Vitro Diagnostics
- Guidance for US Laboratories Testing for Zika Virus Infection (CDC)
- July 15, 2016: HHS Summit to Accelerate Zika Diagnostics Development
- CDC works rapidly to develop unprecedented Zika test (from CDC)


