FDA Sample Collection Criteria and Information for Vaping Related Incidents
CDC and FDA Product Sample Submission Information
- CDC and FDA are working together to coordinate analysis of e-cigarette, or vaping, products to provide insight into the nature of the chemical exposure(s) contributing to the lung injury outbreak.
- As of December 19, CDC and FDA are accepting case-associated product samples for aerosol or e-liquid testing if corresponding bronchoalveolar lavage (BAL) fluid samples are submitted to CDC (samples do not need to be submitted at the same time to meet this requirement). CDC and FDA will no longer be accepting isolated case-associated product samples.
- In e-cigarette, or vaping, product use-associated lung injury (EVALI)-related death cases, CDC and FDA will continue to accept product samples for testing even if no BAL fluid sample is available for testing.
- This change does not mean that the investigation has concluded. The extensive product testing conducted by both FDA and CDC to date has provided critical information regarding potential chemicals of concern. At this time, additional focused testing is needed for paired specimen testing (i.e., both product and BAL fluid samples) in order to continue to further inform the investigation.
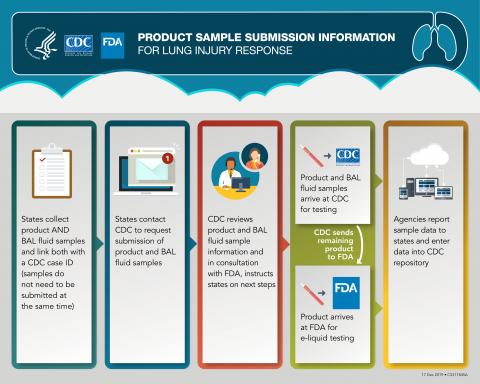
- Additionally, CDC and FDA are streamlining the sample collection process to reduce the burden on state partners. All product samples and corresponding BAL fluid samples will be submitted to CDC. CDC and FDA will coordinate sample testing and transfer to testing laboratories as appropriate.
- CDC will conduct aerosol emissions testing of e-cigarette, or vaping, products. FDA is analyzing e-liquids for the presence of a broad range of chemicals. Analysis of both aerosol emissions and e-liquids will complement each other, and together will help improve our understanding of exposures among case patients associated with the lung injury outbreak.
The graphic below outlines the process for states to submit product samples to CDC for routing and testing in coordination with FDA. Upon receipt of product samples, CDC will determine whether sufficient liquid product is available to perform aerosol and e-liquid analysis and coordinate testing with FDA.
- For information about collection and submission of e-cigarette or other vaping products, including e-liquids, associated with confirmed or probable cases for possible testing by FDA, contact FDAVapingSampleInquiries@fda.hhs.gov.
- For information about collection and submission of e-cigarette or other vaping products’ e-liquids associated with confirmed or probable cases for possible aerosol emissions testing by CDC, contact IncidentResponse@cdc.gov.