Submit Data to GUDID
Device labelers must complete all steps in Prepare for GUDID and then Request a GUDID Account before they submit GUDID data.
The FDA provides device labelers with two options for submitting GUDID data:
- Manual data entry using the GUDID web application: For submitting single device identifier (DI) records manually through GUDID web application online.
- HL7 SPL file submission using FDA Electronic Submissions Gateway: For submitting DI records in XML files that comply with Health Level 7 (HL7) Structured Product Labeling (SPL).
Tip: How to Select the Best Option for Submitting GUDID Data
| GUDID web application |
|
| HL7 SPL File Submission |
|
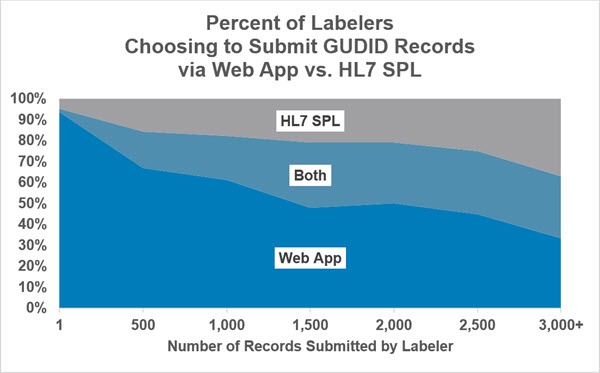
The following graphs from April 2019 illustrate differences in the percentage of labelers using the two options by the number of records submitted as well as the percentage of submissions using the two options.
Based on Number of Records Submitted from May 2014 through April 2019
Based on Number of Records Submitted from May 2014 through April 2019
Option 1: Using the GUDID Web Application to Submit Data Online
The GUDID web application is an online application for submitting GUDID data.
Before you use the GUDID web application, read these user manuals:
- GUDID User Manual (PDF – 2.2MB)
- GUDID User Manual for Unlocking Device Records for Editing (PDF – 427KB)
These manuals provide step-by-step technical instructions and illustrations for using the GUDID web application to submit data.
Option 2: Using the FDA Electronic Submissions Gateway to Submit HL7 SPL Files in XML Format
This option enables companies to electronically submit device information one DI record at a time in an XML file that complies with Health Level 7 (HL7) Structured Product Labeling (SPL).
- Health Level 7 (HL7) is a not for profit, American National Standards Institute (ANSI) accredited standards development organization, whose mission is to provide messaging standards for healthcare interoperability, exchange, management, and integration of data that supports clinical patient care and the management, delivery, and evaluation of healthcare services.
- Structured Product Labeling (SPL) is an HL7 standard for the exchange of product information using extensible markup language (XML). For GUDID, the FDA uses the HL7 SPL Release 5, Draft Standard for Trial Use (DSTU), to receive device identification information.
To use this option:
- Follow the instructions in Prepare for GUDID and then Request a GUDID Account to establish GUDID and FDA Electronic Submissions Gateway accounts.
- After the accounts are established, complete GUDID testing prior to submitting GUDID data. The HL7 SPL Implementation Files provide detailed information on the testing requirements and process. The files have been updated to include GUDID v1.2.3 HL7 SPL Production release information.