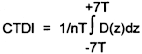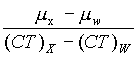GUIDANCE DOCUMENT
Guide for the Submission of Initial Reports on Computed Tomography X-Ray Systems December 1985
This guidance was written prior to the February 27, 1997 implementation of FDA’s Good Guidance Practices, GGP’s. It does not create or confer rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statute, regulations, or both. This guidance will be updated in the next revision to include the standard elements of GGP’s.
A Guide For the Submission of Initial Reports on Computed Tomography X-Ray Systems
PUBLISHED BY:
DIVISION OF STANDARDS ENFORCEMENT
X-RAY PRODUCTS BRANCH
DECEMBER 1985
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
PUBLIC HEALTH SERVICE
FOOD AND DRUG ADMINISTRATION
CENTER FOR DEVICES AND RADIOLOGICAL HEALTH
CONTENTS
Section 102.0 - Product Identification
Section 103.0 - Labeling/Information
PART 200 - SYSTEM DESCRIPTION ...........
Section 202.0 - Control and Indication of the CT Conditions of Operation - Timers
Section 203.0 - Tomographic Plane Indication & Alignment
Sections 204.0 - Beam on & Shutter Status Indicators
Section 205.0 - CT Number Mean & Standard Deviation
Section 206.0 - Labeling
Section 302.0 - Beam Quality
Section 303.0 - Peak Tube Potential
Section 304.0 - Tube Current
Section 305.0 - Scan Time
Section 306.0 - Tube Current - Exposure Time Product
Section 307.0 - CTDI/Dose Profile Information
Section 308.0 - Imaging Performance
Section 309.0 - Equipment Failure Exposure Termination ....
Section 310.0 - Tomographic Plane Location
Section 311.0 - Illumination Levels of the Light Source ...
Section 312.0 - Shutter Leakage Radiation
Section 313.0 - Scan Increment Accuracy
A GUIDE FOR THE SUBMISSION OF INITIAL REPORTS ON COMPUTED TOMOGRAPHY X-RAY SYSTEMS
INTRODUCTION
This guide outlines for a manufacturer, a format for the presentation of initial and supplemental reports on computed tomography (CT) x-ray systems which are subject to the Performance Standard 21 CFR 1020.30 and 1020.33.
The subject reporting guide is an attempt to identify the pertinent information needed by the Center for Devices and Radiological Health (the Center) under the Radiation Control for Health and Safety Act of 1968 (P.L. 90-602). The identification of this information will make the manufacturer's reporting task somewhat easier since, after the initial organization of the material, the manufacturer will not be obligated to prepare and submit such voluminous reports as in the past.
This new guide asks for information in four parts. PART 100 IDENTIFICATION, containing three sections, asks for information with regard to identification, labeling, and user's information. PART 200 - SYSTEM DESCRIPTION, containing five sections, asks for information pertaining to specific performance characteristics of the equipment. Manufacturers must answer all questions in this section, as they relate to their systems. PART 300 - QUALITY CONTROL, containing thirteen sections, asks for presentations of prototype, production, and assembler test methods and results. PART 400 - COMMON ASPECTS, containing two sections, asks for test instrument specifications and sampling protocols. The manufacturer must answer all applicable questions of sections 401.0 and 402.0.
Manufacturers are encouraged to submit a "Common Aspects Report" in order to simplify their reporting obligations. The Common Aspects Report is a separate initial report that incorporates a description of test methods, instrumentation, and sampling plans common to several models. This Common Aspects Report is not intended as a means for certification of any specific model. Currently, separate initial reports from the same manufacturer often provide identical descriptions of the quality control program. Such duplication is costly and entails extra effort for both the manufacturer and the Center. By development of a Common Aspects Report, standardized test methods, instrumentation, and sampling plans may be collected into one report. Initial reports for specific models can reference applicable sections of the Common Aspects Report.
All material shall be submitted in the English language or with an accurate attached English translation. Definitions for technical terms used in this guide may be found on page iii.
DEFINITIONS
As used in this guide and 21 CFR 1020.30 and 1020.33, the following definitions apply:
| (1) | "Assembler" means any person engaged in the business of assembling, replacing, or installing one or more components into an x-ray system or subsystem. | ||||||||||
| (2) | "Beam-limiting device" means a device which provides a means to restrict the dimensions of the x-ray field. | ||||||||||
| (3) | "Computed tomography dose index (CTDI)II means the integral of the dose profile along a line perpendicular to the tomographic plane divided by the product of the nominal tomographic section thickness and the number of tomograms produced in a single scan. This is: | ||||||||||
| Where: | |||||||||||
| z | = Position along a line perpendicular to the tomographic plane. | ||||||||||
| D(z) | = Dose at position z. | ||||||||||
| T | = Nominal tomographic section thickness. | ||||||||||
| n | = Number of tomograms produced in a single scan. | ||||||||||
| This definition assumes that for a multiple tomogram system the scan increment between adjacent scans is nT. | |||||||||||
| (4) | "Contrast scale" means the change in linear attenuation coefficient per CT number relative to water, that is:
| ||||||||||
| Where: | |||||||||||
| µw | = Linear attenuation Coefficient of water. | ||||||||||
| µx | = Linear attenuation coefficient of material of interest. | ||||||||||
| (CT)W | = CT number of water. | ||||||||||
| (CT)X | = CT number of material of interest | ||||||||||
| (5) | "Control panel" means that part of the x-ray control upon which are mounted the switches, knobs, pushbuttons, and other hardware necessary for manually setting the technique factors. | ||||||||||
| (6) | "Cooling curve" means the graphical relationship between heat units stored and cooling time. | ||||||||||
| (7) | "CT conditions of operation" means all selectable parameters governing the operation of a CT system including nominal tomographic section thickness, filtration, and the technique factors as defined in Section 1020.30(b)(36). | ||||||||||
| (8) | "CT number" means the number used to represent the x-ray attenuation associated with each elemental area of the CT image. | ||||||||||
| (9) | "CT dosimetry phantom" means the phantom used for determination of the dose delivered by a computed tomography system. The phantom shall be a right circular cylinder of polymethylmethacrylate of density 1.19 + 0.01 grams per cubic centimeter. The phantom shall be at least 14 centimeters in length and shall have diameters of 32.0 centimeters for testing any CT system designed to image any section of the body (whole body scanners) and 16.0 centimeters for any system designed to image the head (head scanners) or for any whole body scanner operated in the head scanning mode. The phantom shall provide means for the placement of a dosimeter(s) along its axis of rotation and along a line parallel to the axis of rotation 1.0 centimeter from the outer surface and within the phantom. Means for the placement of a dosimeter(s) or alignment device at other locations may be provided for convenience. The means used for placement of a dosimeter(s) (i.e., hole size) and the type of dosimeter(s) used is at the discretion of the manufacturer. Any effect on the doses measured due to the removal of phantom material to accommodate dosimeters shall be accounted for through appropriate corrections to the reported data or included in the statement of maximum deviation for the values obtained using the phantom. | ||||||||||
| (10) | "Diagnostic source assembly" means the tube housing assembly with a beam-limiting device attached. | ||||||||||
| (11) | "Diagnostic x-ray system" means an x-ray system designed for irradiation of any part of the human body for the purpose of diagnosis or visualization. | ||||||||||
| (12) | "Dose profile" means the dose as a function of position along a line. | ||||||||||
| (13) | "Equipment" means x-ray equipment. | ||||||||||
| (14) | "Exposure" means the quotient of dQ by dm where dQ is the absolute value of the total charge of the ions of one sign produced in air when all the electrons (negatrons and positrons) liberated by photons in a volume element of air having mass dm are completely stopped in air. | ||||||||||
| (15) | "Half-value layer, HVL" means the thickness of specified material which attenuates the beam of radiation to an extent such that the exposure rate is reduced to one-half of its original value. In this definition the contribution of all scattered radiation, other than any which might be present initially in the beam concerned, is deemed to be excluded. | ||||||||||
| (16) | "Image receptor" means any device, such as a fluorescent screen or radiographic film or detector array which transforms incident x-ray photons either into a visible image or into another form which can be made into a visible image by further transformations. | ||||||||||
| (17) | "Leakage radiation" means radiation emanating from the diagnostic source assembly except for: | ||||||||||
| (i) | The useful beam and | ||||||||||
| (ii) | radiation produced when the exposure switch or timer is not activated. | ||||||||||
| (18) | "Leakage technique factors" means the technique factors associated with the tube housing assembly which are used in measuring leakage radiation. They are defined as follows: | ||||||||||
| (i) | For tube housing assemblies intended for capacitor energy storage equipment, the maximum-rated peak tube potential and the maximum-rated number of exposures in an hour for operation at the maximum-rated peak tube potential with the quantity of charge per exposure being 10 millicoulombs (mAs) or the minimum obtainable from the unit, whichever is larger. | ||||||||||
| (ii) | For tube housing assemblies intended for field emission equipment rated for pulsed operation, the maximum-rated peak tube potential and the maximum-rated number of x-ray pulses in an hour for operation at the maximum-rated peak tube potential. | ||||||||||
| (iii) | For all other tube housing assemblies, the maximum-rated peak tube potential and the maximum-rated continuous tube current for the maximum-rated peak tube potential. | ||||||||||
| (19) | "Line-voltage regulation" means the difference between the no-load and the load line potentials expressed as a percent of the load line potential; that is, Percent line-voltage regulation = 100(Vn - Vi)/Vi Where:
| ||||||||||
| (20) | "Maximum line current" means the rms current in the supply line of an x-ray machine operating at its maximum rating. | ||||||||||
| (21) | "Modulation transfer function" means the modulus of the Fourier transform of the impulse response of the system. | ||||||||||
| (22) | "Multiple tomogram system" means a computed tomography system which obtains x-ray transmission data simultaneously during a single scan to produce more than one tomogram. | ||||||||||
| (23) | "Noise" means the standard deviation of the fluctuations in CT number expressed as a percent of the attenuation coefficient of water. Its estimate (S n ) is calculated using the following expression:
| ||||||||||
Where:
| |||||||||||
| (24) | "Nominal tomographic section thickness" means the full-width at half-maximum of the sensitivity profile taken at the center of the cross-sectional volume over which x-ray transmission data are collected. | ||||||||||
| (25) | "Peak tube potential" means the maximum value of the potential difference across the x-ray tube during an exposure. | ||||||||||
| (26) | "Picture element" means an elemental area of a tomogram. | ||||||||||
| (27) | "Rated line voltage" means the range of potentials, in volts, of the supply line specified by the manufacturer at which the x-ray machine is designed to operate. | ||||||||||
| (28) | "Rated output current" means the maximum allowable load current of the x-ray high-voltage generator. | ||||||||||
| (29) | "Rated output voltage" means the allowable peak potential, in volts, at the output terminals of the x-ray high-voltage generator. | ||||||||||
| (30) | "Rating" means the operating limits specified by the manufacturer. | ||||||||||
| (31) | "Recording" means producing a permanent form of an image resulting from x-ray photons (e.g., film, videotape). | ||||||||||
| (32) | "Response time" means the time required for an instrument system to reach 90 percent of its final reading when the radiation--sensitive volume of the instrument system is exposed to a step change in radiation flux from zero sufficient to provide a steady state midscale reading. | ||||||||||
| (33) | "Scan increment" means the amount of relative displacement of the patient with respect to the CT x-ray system between successive scans measured along the direction of such displacement. | ||||||||||
| (34) | "Scan sequence" means a preselected set of two or more scans performed consecutively under preselected CT conditions of operation. | ||||||||||
| (35) | "Sensitivity profile" means the relative response of the CT system as a function of position along a line perpendicular to the tomographic plane. | ||||||||||
| (36) | "Single tomogram system" means a CT system which obtains x-ray transmission data during a scan to produce a single tomogram. | ||||||||||
| (37) | "Source" means the focal spot of the x-ray tube. | ||||||||||
| (38) | "Source-image receptor distance (SID)" means the distance from the source to the center of the input surface of the image receptor. | ||||||||||
| (39) | "Stationary equipment" means equipment which is installed in a fixed location. | ||||||||||
| (40) | "Tomogram" means the result of imaging a particular body section or slice. | ||||||||||
| (41) | "Tomographic plane" means that geometric plane which the manufacturer identifies as corresponding to the output tomogram. | ||||||||||
| (42) | "Tomographic section" means the volume of an object whose x-ray attenuation properties are imaged in a tomogram. | ||||||||||
| (43) | "Tube" means an x-ray tube, unless otherwise specified. | ||||||||||
| (44) | "Tube housing assembly" means the tube housing with tube installed. It includes high-voltage and/or filament transformers and other appropriate elements when they are contained within the tube housing. | ||||||||||
| (45) | "Tube rating chart" means the set of curves which specify the rated limits of operation of the tube in terms of the technique factors. | ||||||||||
| (46) | "Useful beam" means the radiation which passes through the tube housing port and the aperture of the beam-limiting device when the exposure switch or timer is activated. | ||||||||||
| (47) | "Variable-aperture beam-limiting device" means a beam-limiting device which has capacity for stepless adjustment of the x-ray field size at a given SID. | ||||||||||
| (48) | "Visible area" means that portion of the input surface of the image receptor over which incident x-ray photons are producing a visible image. | ||||||||||
| (49) | "X-ray control" means a device which controls input power to the x-ray high-voltage generator and/or the x-ray tube. It includes equipment such as timers, phototimers, automatic brightness stabilizers, and similar devices, which control the technique factors of an x-ray exposure. | ||||||||||
| (50) | "X-ray equipment" means an x-ray system, subsystem, or component thereof. | ||||||||||
| (51) | "X-ray field" means that area of the intersection of the useful beam and any one of the set of planes parallel to and including the plane of the image receptor, whose perimeter is the locus of points at which the exposure rate is one-fourth of the maximum in the intersection. | ||||||||||
| (52) | "X-ray high-voltage generator" means a device which transforms electrical energy from the potential supplied by the x-ray control to the tube operating potential. The device may also include means for transforming alternating current to direct current, filament transformers for the x-ray tube(s), high-voltage switches, electrical protective devices, and other appropriate elements. | ||||||||||
| (53) | "X-ray system" means an assemblage of components for the controlled production of X rays. It includes minimally an x-ray high-voltage generator, an x-ray control, a tube housing assembly, a beam-limiting device, and the necessary supporting structures. Additional components which function with the system are considered iptegral parts of the system. | ||||||||||
| (54) | "X-ray tube" means any electron tube which is designed for the conversion of electrical energy into x-ray energy. | ||||||||||
PART 100 - IDENTIFICATION
| 101.0 | REPORT IDENTIFICATIONPlease confirm that this report is submitted pursuant to section 1002.10. State each of the following:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 102.0 | PRODUCT IDENTIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 102.1 | Give the designation of the system being certified in this report:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Identify each certifiable component that is included in the system. Include component type, model designation, and manufacturer (certifiable component types: tube housing assemblies, x-ray controls, x-ray high voltage generators, tables, beam-limiting devices, and gantries.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Note: | The gantry certification label serves as the system certification. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 102.2 | Identify any of the components listed in part 102.1 that you are certifying in this report.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 103.0 | LABELING/INFORMATIONInformation requested under this section should be supplied in the appropriate appendix. Provide an explanation for information that is unavailable or not applicable. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 103.1 | Provide, in Appendix A, copies of the following labels along with a photograph or drawing of each certifiable component and/or system showing the location of the attached label. The standard requires that labels be permanently affixed, legible, and accessible to view. In the case of beam-limiting devices and tube housing assemblies contained within the gantry, the identification and certification labels shall be mounted on the component even though the component is not visible. The gantry certification shall serve as the certifying label for the entire CT system. In addition, the date of manufacture as indicated on the gantry label shall serve as the manufacturing date for the entire CT system.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 103.2 | Provide, in Appendix B, a copy of the assembler information requested below. Indicate the exact page number where this information may be found.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 103.3 | Provide a copy of the users' information listed below. Provide the exact page number of the location of each item within each appendix. All users' information listed below shall be identified and provided in a separate section of the users, instruction manual or in a separate manual devoted only to this information.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PART 200 - SYSTEM DESCRIPTION
For each section listed below, give a complete description of the means provided to satisfy the requirement.
| 201.0 | CONTROL AND INDICATION OF THE CT CONDITIONS OF OPERATION -VISUAL INDICATION |
| 201.1 | All CT conditions of operation must be displayed prior to the initiation of each scan or scan sequence (1020.33(f)(1)). Along with a description of the means provided, you should include a drawing or picture of the preindicators of technique factors to the operator. |
| 201.2 | The displayed conditions of operation must be visible from any position from which scan initiation is possible (1020.33(f)(1)). Provide a drawing or picture that illustrates the proximity of any exposure switch to the preindicated technique factors. |
| 202.0 | CONTROL AND INDICATION OF THE CT CONDITIONS OF OPERATION - TIMERS |
| 202.1 | In the event of equipment failure, means must be provided to automatically limit the total scan time to no more than 110% of its preset value (1020.33(f)(2)(i)). Give a complete description of the backup safety device which is provided for this requirement. |
| 202.2 | Visual indication must be provided to identify scans terminated through these means (1020.33(f)(2)(i)). In addition to a description of the means provided, you should include a picture or drawing of the visible signal that indicates when an exposure has been terminated by the backup safety device. |
| 202.3 | Means must be provided for the manual resetting of the conditions of operation, in the event of equipment failure, prior to the initiation of another scan (1020.33(f)(2)(i)). Describe the manual resetting procedures. |
| 202.4 | Means must be provided such that the exposure from the system does not exceed the radiation levels specified in paragraph 1020-30(k) except when x-ray transmission data are being collected for use in image production or technique factor selection (1020.33(f)(2)(ii)). Give a description of your design which will limit the dose to the patient to only those circumstances stated above. |
| 202.5 | Means must be provided for the operator to terminate the x-ray exposure at any time during a scan, or series of scans of greater than 0.5 seconds duration (1020.33(f)(2)(iii)). Describe this method. |
| 202.6 | Termination of the x-ray exposure, by the operator, must require manual resetting of the conditions of operation prior to initiation of another scan (1020.33(f)(2)(iii)). Describe the manual resetting procedure. |
| 203.0 | TOMOGRAPHIC PLANE INDICATION & ALIGNMENT |
| 203.1 | For any single tomogram, system, means shall be provided to permit visual determination of the tomographic plane or an offset reference plane (1020.33(g)(1)). Describe the specific means utilized for indication of location on the patient where the tomogram will be obtained. |
| 203.2 | For any multiple tomogram system, means must be provided to permit visual determination of the location of a reference plane (1020.33(g)(2)). For multiple tomogram systems, describe the relationship of the reference plane alignment to the actual position of the tomograms. |
| 204.0 | BEAM ON & SHUTTER STATUS INDICATORS |
| 204.1 | Means shall be provided on the x-ray control and on or near the housing of the scanning mechanism to provide visual indication when and only when X rays are produced (1020.33(h)(1)). In addition to a description of this means, provide a drawing or picture to show visual indicators. |
| 204.2 | If applicable, means shall be provided on the x-ray control and on or near the housing of the scanning mechanism to provide visual indication of whether the shutter is open or closed (1020.33(h)(1)). In addition to a description of this means, provide a drawing or picture to show the visual indicators. |
| 204.3 | The minimum period for x-ray on indication must be 0.5 seconds or greater (1020.33(h)(1)). Describe the means provided to meet this requirement. |
| 204.4 | Visual indicators (indicating x-ray production and shutter status) on or near the housing of the scanning mechanism shall be discernible from any point external to the patient opening, where insertion of any part of the human body into the primary beam is possible (1020.33(h)(1)). In addition to the description of this means, provide a drawing or picture that illustrates the location of all indicators at or near the housing of the scanning mechanism, in relation to the patient opening. |
| 205.0 | CT NUMBER MEAN & STANDARD DEVIATION |
| 205.1 | Means must be provided for the user to calculate the mean and standard deviation of CT numbers for an array of picture elements about any location in the image (1020.33(j)(1)). Describe this means. |
| 205.2 | The number of elements in this array must be under user control (1020.33(j)(1)). Describe the means provided to the user for varying the number of elements in the array. |
| 206.0 | LABELING |
| 206.1 | The warning label must be legible and clearly visible on the control panel containing the main power switch (1020.30(j)). |
| 206.2 | The identification label must contain the name & address of the manufacturer (or the individual or company under whose name it was sold), the place of manufacture, & the model designation and serial number (1010.3(a)(1)(2)). |
| 206.3 | The month and year of manufacture must be provided clearly & legibly without abbreviation, and with the year shown as a four-digit number follows: manufactured: (insert month and year of manufacture) (1010.3(a)(2)(ii)). |
| 206.4 | If the place of manufacture as stated on the identification label is coded, please provide that code (1010.3(a)(2)(i)). |
PART 300 - QUALITY CONTROL
For each applicable test listed below, verify that the testing adequately reflects the critical parameters and addresses the "worst case" conditions. As a result of inherent inaccuracies of the test methods and instrumentation, rejection limits for any test must be sufficiently restrictive to account for these inaccuracies.
| 301.0 | LEAKAGE RADIATION FROM THE DIAGNOSTIC SOURCE ASSEMBLY | |
| 301.1 | Requirement The leakage radiation from the diagnostic source assembly measured at distance of 1 meter in any direction from the source shall not exceed 100 milliroentgens in 1 hour when the x-ray tube is operated at its leakage technique factors. Compliance shall be determined by measurements averaged over an area of 100 square centimeters with no linear dimension greater than 20 centimeters (1020.30(k)). | |
| 301.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | The test results must include data representative of each compatible combination of tube housing assembly, beam-limiting device, and gantry. | |
| b. | To assure the use of maximum rated peak tube potential and continuous tube current, the test method(s) must provide the procedure for periodic calibration of technique factors. | |
| c. | For any test using a scan of the diagnostic source assembly, the rate of scan specified in the test method(s) must account for the response time of the radiation instrumentation. | |
| d. | Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 301.3 - 301.5 Provide information as outlined in Attachment A. | ||
| 302.0 | BEAM QUALITY | |
| 302.1 | Requirement The half-value layer of the useful beam for a given x-ray tube potential shall not be less than the values shown in Table I of the diagnostic x-ray standard (see 1020.30(m)). | |
| 302.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | The test results must include data representative of each compatible combination of tube housing assembly and beam-limiting device. | |
| b. | Since the peak tube potential has a critical effect on determining the half-value layer, the test method(s) must provide the procedure for periodic calibration of tube potential. | |
| c. | To minimize the effect of scatter radiation, the x-ray field specified in the test method(s) must be just large enough to cover the sensitive volume of the detector. | |
| d. | Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 302.3 - 302.5 Provide information as outlined in Attachment A. | ||
| 303.0 | PEAK TUBE POTENTIAL | |
| 303.1 | Requirement The manufacturer shall state the maximum deviation of the peak tube potential from its preindicated value during an exposure when the equipment is connected to an adequate power supply as specified by the manufacturer. The deviation of the pe4 tube potential shall not exceed the limits given (see 1020.30(h)(3)(vi)). | |
| 303.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | To assure compliance with the maximum deviation statements provided to the user, the test results must include data for "worst case" combinations of technique factors and supply line conditions (e.g., highest kW, minimum, and maximum allowable line-voltage regulation). | |
| b. | Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 303.3 - 303.5 Provide information as outlined in Attachment A. | ||
| 304.0 | TUBE CURRENT | |
| 304.1 | Requirement The manufacturer shall state the maximum deviation of the tube current from its preindicated value during an exposure, when the equipment is connected to an adequate power supply as specified by the manufacturer. The deviation of the tube current shall not exceed the limits given (see 1020.30(h)(3)(vi)). | |
| 304.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | To assure compliance with the maximum deviation statements provided to the user, the test results must include data for "worst case" combinations of technique factors and supply line conditions (e.g., highest kW, minimum, and maximum allowable line-voltage regulation). | |
| b. | Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 304.3 - 304.5 Provide information as outlined in Attachment A. | ||
| 305.0 | SCAN TIME | |
| 305.1 | Requirement The manufacturer shall state the maximum deviation of the scan time from its preindicated value during an exposure, when the equipment is connected to an adequate power supply as specified by the manufacturer. The deviation of scan time shall not exceed the limits given (see 1020.30(h)(3)(vi)). | |
| 305.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | To assure compliance with the maximum deviation statements provided to the user, the test results must include data for "worst case" combinations of technique factors and supply line conditions (e.g., highest kW, minimum and maximum allowable line-voltage regulation). | |
| b. | Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 305.3 - 305.5 Provide information as outlined in Attachment A. | ||
| 306.0 | TUBE CURRENT - EXPOSURE TIME PRODUCT | |
| 306.1 | Requirement The manufacturer shall state the maximum deviation of the tube current exposure time product (mAs) from its preindicated value during an exposure, when the equipment is connected to an adequate power supply as specified by the manufacturer. The deviation of the tube current exposure time product shall not exceed the limits given (see 1020.30(h)(3)(vi)). | |
| 306.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | To assure compliance with the maximum deviation statements provided to the user, the test results must include data for "worst case" combinations of technique factors and supply line conditions (e.g., highest kW, minimum and maximum allowable line-voltage regulation). | |
| b. | Please note and describe any critical parameters and "worst case', conditions which are unique to your system or test method. | |
| 306.3 - 306.5 Provide information as outlined in Attachment A. | ||
| 307.0 | CTDI/DOSE PROFILE INFORMATIONIndicate for each modality, e.g., head, body, or spine procedure: | |
| a. | A statement of the typical scan technique factors (e.g., kVp, mAs, pulse width, time, etc.) | |
| b. | A statement of the scan diameter. | |
| c. | A statement of the system slice thicknesses. | |
| d. | A statement of the accuracy of the parameters indicated above. | |
| e. | A statement indicating whether the maximum CTDI is obtained from integration of the dose profile for a single scan or from a direct measurement of the average dose in an interval equal to the slice thickness at the center of a series of 14 scans that are spaced by the nominal tomographic slice thickness. | |
| f. | A statement of accuracy of the exposure measurement. | |
| 307.1 | Requirement The manufacturer shall state the maximum deviation of the dose values given to the user in accordance with sections 1020.33(c)(2)(i), (ii), (iii), and (iv). The deviation from these values shall not exceed the limits given (1020.33(c)(2)(v)). | |
| 307.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | All dose measurements must be performed with the CT dosimetry phantom placed on the patient couch or support device without additional attenuating materials present. | |
| b. | The CT conditions of operation for obtaining the CTDI at the five specified locations shall correspond to typical values (e.g., kVp, mAs, scan diameter slice thickness) suggested by the manufacturer for CT of the head, body, or spine as may be appropriate. | |
| c. | The normalized CTDI values must be at least the minimum, maximum mid-range values for the condition of operation or the values available with the other conditions of operation set at the typical values. | |
| d. | Please note any assumptions made in or limitations of your test methods in determining the dose values for your system. | |
| 307.3 - 307.5 Provide information as outlined in Attachment A. | ||
| 308.0 | IMAGING PERFORMANCE | |
| 308.1 | Requirement The manufacturer shall state the maximum deviation from the specifications regarding imaging performance provided in accordance with section 1020.33(c)(3)(i), (ii), (iii), and (iv). The deviation from these values shall not exceed the limits given (1020.33(c)(3)(v)). Questions in this section should be answered as they relate to each of the items listed in the specified paragraphs of 1020.33(c)(3). | |
| 308.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | The CT conditions of operation shall correspond to those us( 1020.33(c)(2)(i), the typical conditions of operation suggel the manufacturer for CT of the head, body, or spine as may appropriate. | |
| b. | All aspects of data collection including the x-ray attenuat properties of the material in the tomographic section shall similar to those used to provide the dose information requi section 1020.33(c)(2)(i). | |
| c. | Please note any assumptions made in, or limitations of, the methods in determining the imaging parameters. | |
| 308.3 - 308.5 Provide information as outlined in Attachment A. | ||
| 308.3 - 308.5 See Attachment A. | ||
| 309.0 | EQUIPMENT FAILURE EXPOSURE TERMINATION | |
| 309.1 | Requirement Means shall be provided to terminate the x-ray exposure automatically by either deenergizing the x-ray source or shuttering the x-ray beam in the event of equipment failure affecting data collection. Such termination shall occur within an interval that limits the total scan time to no more than 110 percent of its preset value through the use of either a backup timer or devices which monitor equipment function (1020.33(f)(2)(i)). | |
| 309.2 | Critical Parameters and "Worst Case" Conditions Please note and describe any critical parameters and "worst conditions which are unique to your system or test method. | |
| 309.3 - 309.5 Provide information as outlined in Attachment A. | ||
| 310.0 | TOMOGRAPHIC PLANE LOCATION | |
| 310.1 | Requirement The distance between the indicated location of the tomographic plane or reference plane and its actual location shall not exceed 5 millimeters (1020.33(g)(3)). | |
| 310.2 | Critical Parameters and "Worst Case" Conditions Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 310.3 - 301.5 Provide information as outlined in Attachment A. | ||
| 311.0 | ILLUMINATION LEVELS OF THE LIGHT SOURCE | |
| 311.1 | Requirement If a device using a light source is used to satisfy the requirements of paragraph 1020.33(g)(1) & (2), the light source shall permit visual determination of the location of the tomographic plane or reference plane under ambient light conditions of up to 500 lux (1020.33(g)(5)). | |
| 311.2 | Critical Parameters and "Worst Case" Conditions Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 311.3 - 311.5 Provide information as outlined in Attachment A. | ||
| 312.0 | SHUTTER LEAKAGE RADIATION | |
| 312.1 | Requirement For systems that allow high voltage to be applied to the x-ray tube continuously and that control the emission of x-rays with a shutter, the radiation emitted shall not exceed 100 milliroentgens (2.58 x 10-5 coulomb/kilogram) in 1 hour at any point 5 centimeters outside the external surface of the housing of the scanning mechanism when the shutter is closed. Compliance shall be determined by measurements averaged over an area of 100 square centimeters with no linear dimension greater than 20 centimeters (1020.33(h)(2)). | |
| 312.2 | Critical Parameters and "Worst Case" Conditions | |
| a. | For any test using a scan of the diagnostic source assembly, the rate of scan specified in the test method(s) must account for the response time of the radiation instrumentation. | |
| b. | Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 312.3 - 312.5 Provide information as outlined in Attachment A. | ||
| 313.0 | SCAN INCREMENT ACCURACY | |
| 313.1 | Requirement The deviation of indicated scan increment from actual scan increment shall not exceed 1 mm. Compliance shall be measured as follows: The determination of the deviation of indicated versus actual scan increment shall be based on measurements taken with a mass, less than or equal to 100 kilograms, on the patient support device. The patient support device shall be incremented from a typical starting position to the maximum incrementation distance or 30 centimeters, whichever is less, and then returned to the starting position. Measurement of actual versus indicated scan increment may be taken anywhere along this travel (1020.33(i)). | |
| 313.2 | Critical Parameters and "Worst Case" Conditions Please note and describe any critical parameters and "worst case" conditions which are unique to your system or test method. | |
| 313.3 - 313.5 Provide information as outlined in Attachment A. | ||
ATTACHMENT A
The information below is applicable to Part 300.
For each section answered in Part 300 provide the following information. Clearly relate the response to the subsection of Part 300 to which it is applicable. For example, the responses to Section 301.0 should be labeled 301.3 thru 301.5, as appropriate.
| 30X-3 | Prototype Testing | |
| a. | Provide a description of the direct test method (i.e., one that directly measures the parameter in question) employed in testing and/or measuring the parameter for each model with respect to this requirement. | |
| b. | Identify the instrument(s) used for the test by manufacturer and model number. Answer the appropriate section in Part 400 for this instrument(s). | |
| c. | Provide sample raw test data. | |
| d. | If the actual compliance value is calculated from the raw test data, provide a sample of calculated compliance values complete with an explanation of any correction factors employed. | |
| e. | A statement indicating whether the maximum CTDI is obtained from integration of the dose profile for a single scan or from a direct measurement of the average dose in an interval equal to the slice thickness at the center of a series of 14 scans that are spaced by the nominal tomographic slice thickness. | |
| 30X-4 | Production Testing | |
| a. | Describe all methods employed in direct and indirect testing of each model with respect to this requirement. | |
| b. | If an indirect test is used to measure compliance, explain why it is an accurate indication of compliance with this requirement. | |
| c. | Submit the technical data that supports the use of the test in part b. | |
| d. | Provide a copy of the detailed instructions for performing each test. Attach as APPENDIX F. | |
| e. | Identify the instrument(s) used for each test by manufacturer and model number. Answer the appropriate section in Part 400 for each instrument(s). | |
| f. | For each of the above test methods give the page number of your detailed instructions for performing the test and indicate where the rejection limits are specified. | |
| g. | For each of the above test methods, provide sample raw test data. | |
| h. | If the actual compliance value is calculated from the raw test data, provide a sample of calculated compliance values complete with an explanation of any correction factors employed. | |
| i. | If you do not test 100 percent of the produced models, answer the questions in Section 402.0, Part 400. | |
| j. | A statement indicating whether the maximum CTDI is obtained from integration of the dose profile for a single scan or from a direct measurement of the average dose in an interval equal to the slice thickness at the center of a series of 14 scans that are spaced by the nominal tomographic slice thickness. | |
| 30X-5 | Assembler Testing | |
| a-i. | If test instructions are provided to the assembler, answer the questions in 30X.4 with respect to assembler testing. Note: The information requested in 30X.5 (d) (i.e., a copy of detailed instructions for performing each test) should have already been provided in APPENDIX B and thus may be referenced by indicating the appropriate page numbers. | |
PART 400 - COMMON ASPECTS
| 401.0 | INSTRUMENTATION | |
| 401.1 | Radiation Measurement | |
| a. | Describe each radiation measurement instrument that you refer to in Part 300, giving the following: manufacturer and model number if the instrument is commercially available; type of instrument; precision; accuracy; response time; energy dependence; angular response; exposure rate dependence; ranges; and effective measurement area. | |
| b. | Describe the procedures used for calibration of each instrument including the interval of time between calibrations. | |
| c. | How do you assure proper day-to-day operation of each instrument? | |
| 401.2 | Illuminance | |
| a. | Describe each illuminance measurement instrument that you refer to in Part 300, giving the following: manufacturer and model number if the instrument is commercially available; type of instrument; precision; accuracy; and ranges. | |
| b. | Describe the procedures used for calibration of each instrument including the interval of time between calibrations. | |
| c. | How do you assure proper day-to-day operation of each instrument? | |
| 401.3 | Electrical Measurement | |
| a. | Describe each electrical measurement instrument that you referred to in Part 300, giving the following: type of instrument; manufacturer and model number if the instrument is commercially available; rated accuracy; precision; ranges; and response time. If any number of commercially available instrument with certain basic characteristics may be used, it is sufficient to state the minimum accuracy, precision, ranges, response time, and so forth, of the class of instruments that will be used. If any instrument is unique or of special manufacture, then the manufacturer and model number should be stated. | |
| b. | Describe the procedures used for calibration of each instrument including the interval of time between calibrations. | |
| c. | Show where each instrument listed in 401.3(a) is connected during testing with the use of schematic diagrams | |
| 401.4 | Other Measurements | |
| a. | Describe each measurement instrument (other than radiation, illuminance or electrical) that you refer to in Part 300, giving the following: type of instrument; manufacturer and model number if the instrument is commercially available; rated accuracy; precision; and ranges. If any number of commercially available instruments with certain basic characteristics may be used, it is sufficient to state the minimum accuracy, precision ranges, and so forth, of the class of instruments that will be used. If any instrument is unique or of special manufacture, however, then the manufacturer and model number should be stated. | |
| b. | Describe the procedures used for calibration of each instrument including the interval of time between calibrations. | |
| 402.0 | SAMPLING | |
| 402.1 | Description | |
| a. | Describe the sampling plan used and provide the parameters of the plan (e.g., lot size, sample size, rejection criterion). | |
| b. | Describe the procedure used for selecting the sample and indicate how randomness is assured. | |
| c. | Describe the action taken if the sampling plan leads to a rejection decision. | |
Submit Comments
Submit comments on this guidance document electronically via docket ID: FDA-2013-S-0610 - Specific Electronic Submissions Intended For FDA's Dockets Management Staff (i.e., Citizen Petitions, Draft Proposed Guidance Documents, Variances, and other administrative record submissions)
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All comments should be identified with the title of the guidance.