Breakthrough Devices Program
On this page:
- What is the Breakthrough Devices Program?
- What are the benefits of the Breakthrough Devices Program?
- Is my device eligible?
- When to request a Breakthrough Devices Designation
- How to request a Breakthrough Devices Designation
- What to include in a request for a Breakthrough Devices Designation
- When will I find out if my device received a Breakthrough Device Designation
- What a sponsor can expect from the FDA if the Breakthrough Devices Designation is granted
- Are there related programs designed to expedite the availability of certain devices that might apply to my device?
- Will FDA announce when a device has been granted Breakthrough Device designation?
- Breakthrough Devices Program Metrics
- CDRH and CBER Breakthrough Device Marketing Authorizations
- Guidances related to Breakthrough Devices Program
- Contact Us
What is the Breakthrough Devices Program?
The Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to these medical devices by speeding up their development, assessment, and review, while preserving the statutory standards for premarket approval, 510(k) clearance, and De Novo marketing authorization, consistent with the Agency's mission to protect and promote public health.
The Breakthrough Devices Program replaces the Expedited Access Pathway and Priority Review for medical devices. The FDA considers devices granted designation under the Expedited Access Pathway to be part of the Breakthrough Devices Program.
What are the benefits of the Breakthrough Devices Program?
The Breakthrough Devices Program offers manufacturers an opportunity to interact with the FDA's experts through several different program options to efficiently address topics as they arise during the premarket review phase, which can help manufacturers receive feedback from the FDA and identify areas of agreement in a timely way. Manufacturers can also expect prioritized review of their submission. Learn more about the Breakthrough Devices Program principles and features in Sections II and IV of the Breakthrough Devices Program final guidance.
Is my device eligible?
Devices subject to premarket approval applications (PMAs), premarket notification (510(k)) or requests for De Novo designation are eligible for breakthrough device designation if both of the following criteria are met:
| Criteria | Description | Refer to Guidance |
|---|---|---|
| First Criterion | The device provides for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions | Section III.B.1 |
| Second Criterion | The device also meets at least one of the following: | |
|
Section III.B.2.a | |
|
Section III.B.2.b | |
|
Section III.B.2.c | |
|
Section III.B.2.d |
When to request a Breakthrough Devices Designation
You can send a Breakthrough Designation request for your device at any time prior to sending your marketing submission (for example, premarket approval (PMA), premarket notification (510(k)), or De Novo classification request).
How to request a Breakthrough Devices Designation
You can request the Breakthrough Device designation by submitting a "Designation Request for Breakthrough Device" Q-Submission. Your designation request should be the only request in the Q-Submission. If you are pursuing the Breakthrough Device designation while you have other requests for feedback pending, you may want to send the requests for feedback after the FDA makes a designation decision because the designation may affect the feedback that the FDA provides on your other requests. The procedures for submitting a Q-Submission are outlined in the guidance Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program.
The FDA may find devices that could be good candidates for the Breakthrough Devices Program and recommend that sponsors of such devices consider applying to the program.
What to include in a request for a Breakthrough Devices Designation
The FDA recommends that your designation request include information to describe the device, the proposed indication for use, regulatory history, how your device meets the statutory criteria for a Breakthrough Device, and what type of marketing submission you plan to submit to the FDA for your device. Learn more about what to include in your request in Appendix 1 of the Breakthrough Devices Program final guidance.
When will I find out if my device received Breakthrough Device Designation
The FDA intends to request any other information needed to inform the Breakthrough Device designation decision within 30 days of receiving your request. You can expect to receive a letter communicating the FDA's decision to grant or deny the Breakthrough Device designation request within 60 calendar days of the FDA receiving your request.
It is helpful when a sponsor is available and responsive to the FDA's requests throughout the review timeline. If the FDA does not receive the other information needed to decide on a designation request promptly, it may result in denial of the Breakthrough Device designation request.
What a sponsor can expect from FDA if Breakthrough Designation is Granted
If your device is granted the Breakthrough Device Designation, you can choose to interact with the FDA to obtain feedback on your device development through a variety of options including sprint discussions, request for discussion on a data development plan, and request for clinical protocol agreement. Learn more about these options in Section IV of the Breakthrough Devices Program guidance.
You will also receive prioritized review on future regulatory submissions, including Q-Submissions, Investigational Device Exemption (IDE) applications, and marketing submissions.
Are there related programs designed to expedite the availability of certain devices that might apply to my device?
If your device is not eligible for a Breakthrough Device Designation because it is not intended for the treatment or diagnosis of a life-threatening or irreversibly debilitating human disease or condition, you may consider whether or not it would be a candidate for the Safer Technologies Program.
Will the FDA announce when a device has been granted Breakthrough Device designation?
Prior to marketing authorization, the FDA generally cannot publicly disclose whether a sponsor has submitted a Breakthrough Device designation request for a device, or whether FDA has granted or denied the request unless the sponsor decides to make that information available to the public. Additionally, the FDA plans to maintain a list of devices granted Breakthrough Device designation on its webpage, adding devices to the list once the device has received marketing authorization.
Breakthrough Devices Program Metrics
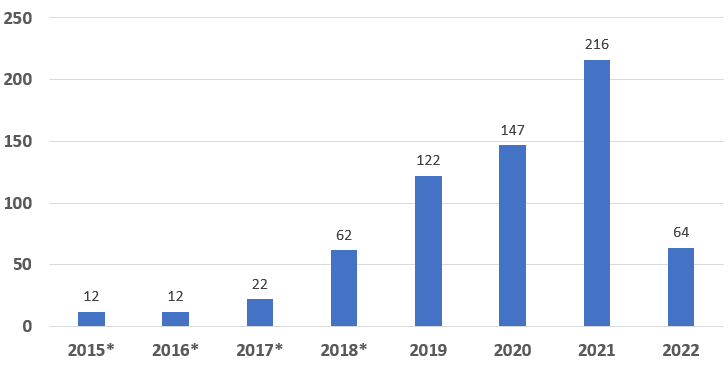
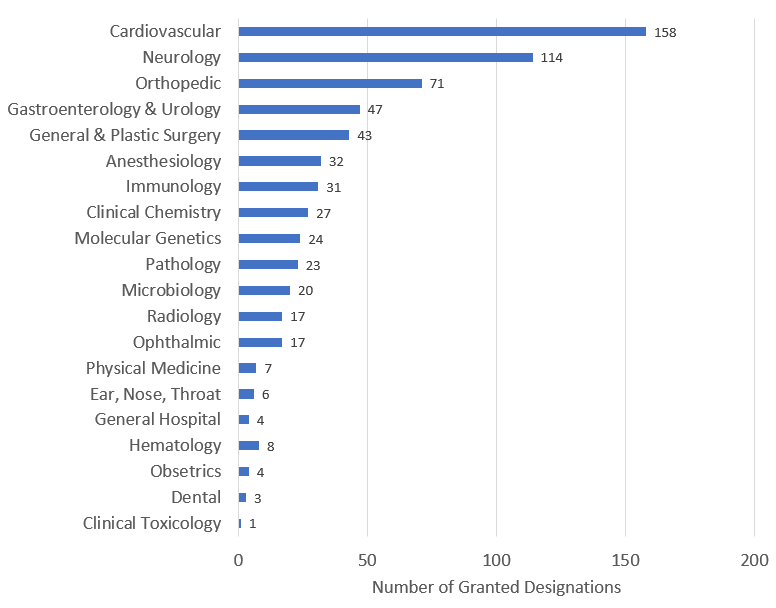
As of March 31, 2022, CDRH and CBER have granted 657 Breakthrough Device designations, including devices originally designated under the Expedited Access Pathway (EAP) program. The following graphs provide the distribution of these designations by fiscal year as well as by clinical panel.
Graph 1: Number of Granted Breakthrough Device Designations by Fiscal Year
Of the 657 devices granted Breakthrough Device designation, CDRH has granted 652 and CBER has granted 5.
* Data includes devices that were designated under the precursor Expedited Access Pathway (EAP). Due to consistency in vision and designation criteria between the precursor EAP Program and the Breakthrough Devices Program, FDA considers devices granted designation under the EAP to be part of the Breakthrough Devices Program.
Graph 2: Number of Granted Breakthrough Device Designations by Clinical Panel
CDRH and CBER Breakthrough Device Marketing Authorizations
Below is a list of CDRH and CBER Breakthrough Devices that have obtained marketing authorization.
CDRH and CBER Breakthrough Device Marketing Authorizations
Data as of March 31, 2022
Total of 44 Marketing Authorizations, including 42 CDRH devices and 2 CBER devices
| Manufacturer | Trade Name | Marketing Submission Number | Marketing Submission Decision Date |
|---|---|---|---|
| CARTIHEAL, LTD. | AGILI-C | P210034 | 03/29/2022 |
| SPECTRANETICS, INC. | CAVACLEAR LASER SHEATH | DEN210024 | 12/21/2021 |
| KOIOS MEDICAL, INC. | KOIOS DS | K212616 | 12/16/2021 |
| APPLIEDVR, INC. | EASEVRX | DEN210014 | 11/16/2021 |
| SYNCTHINK, INC. | EYE-SYNC | K202927 | 10/02/2021 |
| CANARY MEDICAL, INC. | CANARY TIBIAL EXTENSION WITH CANARY HEALTH IMPLANTED REPORTING PROCESSOR (CHIRP) SYSTEM | DEN200064 | 08/27/2021 |
| MICROTRANSPONDER, INC. | VIVISTIM PAIRED VNS SYSTEM | P210007 | 08/27/2021 |
| SIEMENS HEALTHCARE DIAGNOSTICS, INC. | ADVIA CENTAUR ENHANCED LIVER FIBROSIS (ELF) | DEN190056 | 08/20/2021 |
| CARLSMED, INC. | APREVO TRANSFORAMINAL IBF | K210542 | 06/30/2021 |
| COGNOA, INC. | COGNOA ASD DIAGNOSIS AID | DEN200069 | 06/02/2021 |
| PAIGE.AI | PAIGE PROSTATE | DEN200080 | 09/21/2021 |
| NEUROLUTIONS, INC. | NEUROLUTIONS IPSIHAND UPPER EXTREMITY REHABILITATION SYSTEM | DEN200046 | 04/23/2021 |
| HELIUS MEDICAL, INC. | PORTABLE NEUROMODULATION STIMULATOR (PONS) | DEN200050 | 03/26/2021 |
| MEDTRONIC, INC. | HARMONY TPV SYSTEM | P200046 | 03/26/2021 |
| SHOCKWAVE MEDICAL, INC. | SHOCKWAVE INTRAVASCULAR LITHOTRIPSY (IVL) SYSTEM WITH SHOCKWAVE C2 CORONARY INTRAVASCULAR LITHOTRIPSY (IVL) CATHETER | P200039 | 02/12/2021 |
| ABBOTT LABORATORIES | I-STAT ALINITY SYSTEM | K201778 | 01/08/2021 |
| CARLSMED, INC. | APREVO INTERVERTEBRAL BODY FUSION DEVICE | K202034 | 12/03/2020 |
| CERUS CORPORATION | INTERCEPT BLOOD SYSTEM FOR PLASMA | BP130076/S034 | 11/24/2020 |
| NIGHTWARE, INC. | NIGHTWARE KIT | DEN200033 | 11/06/2020 |
| FOUNDATION MEDICINE, INC. | FOUNDATIONONE LIQUID CDX | P200006 | 10/26/2020 |
| ROCHE MOLECULAR SYSTEMS, INC. | COBAS BKV | K202215 | 09/02/2020 |
| MEDTRONIC MINIMED, INC. | MiniMed 770G System | P160017/S076 | 08/31/2020 |
| FOUNDATION MEDICINE, INC. | FOUNDATIONONE LIQUID CDx | P190032 | 08/26/2020 |
| GUARDANT HEALTH, INC. | GUARDANT360 CDx | P200010 | 08/07/2020 |
| ROCHE MOLECULAR SYSTEMS, INC. | COBAS EBV | DEN200015 | 07/30/2020 |
| AMBU INNOVATION GMBH | AMBU DUODENO SYSTEM | K201098 | 07/17/2020 |
| BAY LABS, INC. | CAPTION GUIDANCE | DEN190040 | 02/07/2020 |
| BOSTON SCIENTIFIC | EXALT MODEL D, SINGLE-USE DUODENOSCOPE, EXALT CONTROLLER | K193202 | 12/13/2019 |
| TUSKER MEDICAL | TULA SYSTEM | P190016 | 11/25/2019 |
| ORASURE TECHNOLOGIES | ORAQUICK EBOLA RAPID ANTIGEN TEST | DEN190025 | 10/10/2019 |
| CVRX, INC. | BAROSTIM NEO SYSTEM | P180050 | 08/16/2019 |
| IMPULSE DYNAMICS, INC. | OPTIMIZER SMART SYSTEM | P180036 | 03/21/2019 |
| PEAR THERAPEUTICS | RESET-O | K173681 | 12/10/2018 |
| SPIRATION, INC. | SPIRATION VALVE SYSTEM | P180007 | 12/03/2018 |
| AVITA MEDICAL, LLC. | RECELL AUTOLOGOUS CELL HARVESTING DEVICE | BP170122 | 09/20/2018 |
| PULMONX CORPORATION | ZEPHYR ENDOBRONCHIAL VALVE SYSTEM | P180002 | 06/29/2018 |
| MEDTRONIC MINIMED, INC. | MINIMED 670G SYSTEM | P160017/S031 | 06/21/2018 |
| CLINICAL RESEARCH CONSULTANTS, INC. | CUSTOMFLEX ARTIFICIAL IRIS | P170039 | 05/30/2018 |
| IDX, LLC | IDX-DR | DEN180001 | 04/11/2018 |
| CONCENTRIC MEDICAL, INC. | TREVO PRO VUE RETRIEVER AND TREVO XP PRO VUE RETRIEVER (TREVO RETRIEVER) | K173352 | 02/15/2018 |
| BANYAN BIOMARKERS, INC. | BANYAN BTI | DEN170045 | 02/14/2018 |
| EMPATICA SRL | EMBRACE | K172935 | 01/26/2018 |
| FOUNDATION MEDICINE, INC. | FOUNDATIONONE CDX | P170019 | 11/30/2017 |
| INSIGHTEC | EXABLATE | P150038 | 07/11/2016 |
Guidances related to Breakthrough Devices
- Breakthrough Devices Program Final Guidance
- Factors to Consider When Making Benefit-Risk Determinations in Medical Device Premarket Approval and De Novo Classifications (Benefit Risk Final Guidance)
- Benefit-Risk Factors to Consider When Determining Substantial Equivalence in Premarket Notifications (510(k)) with Different Technological Characteristics - Guidance for Industry and Food and Drug Administration Staff
- Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program
Contact Us
For any questions about the Breakthrough Devices program, please contact BreakthroughDevicesProgram@fda.hhs.gov.