Smisson-Cartledge Biomedical, LLC Recalls ThermaCor 1200 Disposable Sets for Risk of Patient Contact to Aluminum
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product
- ThermaCor 1200 Disposable Sets
- Disposable Set Models: PTC-1200, DNC-1200, PNC-1200
- Distribution Dates: 2006 to present
- Devices Recalled in the U.S.: 38,786
- Date Initiated by Firm: February 18, 2021
Device Use
The ThermaCor 1200 Rapid Thermal Infusion System disposable sets are part of the ThermaCor 1200 Rapid Thermal Infusion System, which is used for fluid or bolus delivery. The system is made of a footswitch for hands-free fluid control, a fluid delivery device, and a single-use disposable set with supply lines that can be used with intravenous (IV) bags or surgical equipment.
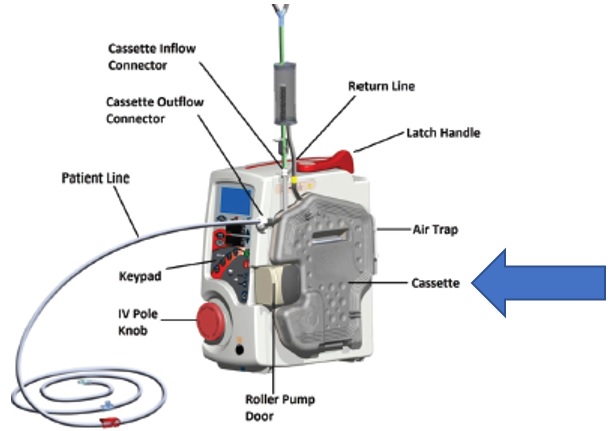
Fig. 1: Image of recalled cassette, which is part of the ThermaCor 1200 Disposable Set
Reason for Recall
Smisson-Cartledge Biomedical is recalling their ThermaCor 1200 Rapid Thermal Infusion System Disposable Sets because a part of the ThermaCor 1200 Disposable Set, the cassette (See Figure 1), which warms fluids directly with an aluminum plate may leak aluminum into the fluids and expose patients to high levels of the metal. The recall is specific to the disposable cassette portion of the device, not to the full pump. Use of the affected product could cause increase exposure to aluminum ions. Exposure to high levels of aluminum ions could cause serious patient harm such as bone or muscle pain and weakness, anemia, seizures, or coma.
There have been no complaints, reports of injuries, or deaths related to this device issue.
Who May be Affected
- Health care providers using the affected ThermaCor 1200 Rapid Thermal Infusion System and ThermaCor 1200 Disposable Sets
- Patients who require care using the affected ThermaCor 1200 Rapid Thermal Infusion System and ThermaCor 1200 Disposable Sets
What to Do
On February 18, 2021, Smisson-Cartledge Biomedical sent an Urgent Medical Device Correction Notification letter to all affected distributors and customers. The letter gave the following information:
- The use of balanced electrolyte solutions (i.e. lactated ringer's solution) should be limited to less than 6 hours.
- Each ThermaCor Cassette is to be used for a maximum total fluid volume up to 25 liters or up to a maximum of 12 consecutive hours, whichever occurs first.
- Higher aluminum levels may occur when using lower flow rates, increased temperatures, with certain fluids, and in longer durations. It also identifies the following patient populations who are especially at risk:
- Neonates, infants, pregnant mothers, and the elderly
- Patients with poor renal function or on dialysis
- Evaluate the benefits and risks of using the device versus the patient condition.
On March 23, 2021, Smisson-Cartledge Biomedical sent a follow up Urgent Medical Device Correction Notification letter to all affected distributors and customers. The letter gave the following information:
To Customers:
- Remove the enclosed laminated card and lanyard from the package and insert the lanyard into the laminated card.
- Using the open end of the lanyard, attach the laminated card to pole clamp knob on the ThermaCor 1200 Rapid Thermal Infuser.
- Complete the enclosed stamped postcard and return to the address at the front of the card.
- Receive acknowledgement that the letter has been reviewed and understood.
- Include the ThermaCor 1200 Rapid Thermal Infuser Serial Number(s) of the devices on the "Acknowledgement Form" that now have the laminated card attached to the unit.
- Sign the card prior to mailing (or e-mailing) back to the company.
- No return of the product is necessary.
To Distributors:
- Review inventory of ThermaCor 1200 Rapid Thermal Infuser units
- Remove the enclosed laminated card and lanyard from the package and insert the lanyard into the laminated card.
- Using the open end of the lanyard, attach the laminated card to pole clamp knob on the ThermaCor 1200 Rapid Thermal Infuse.
- Complete the "Acknowledgement Form" on page four of the Urgent Medical Device Correction Notification letter.
- Receive acknowledgement that the letter has been reviewed and understood.
- Include the ThermaCor 1200 Rapid Thermal Infuser Serial Number(s) of the devices on the "Acknowledgement Form" that now have the laminated card attached to the unit.
- Sign and date the "Acknowledgement Form" prior to mailing (or e-mailing) back to the company.
- Forward information to affected customers or hospitals
- Receive acknowledgement from customers or hospitals that the Urgent Medical Device Correction Notification letter has been reviewed and understood.
- Forward required information, such as signatures and ThermaCor 1200 Rapid Thermal Infuser Serial Number(s), from customers or hospitals to Smisson-Cartledge Biomedical.
- No return of the product is necessary.
Contact Information
Customers who need additional information about this recall can contact Smisson-Cartledge Biomedical by phone at 478-330-6203 or online at [email protected].
Additional Resources
- Recall Database Entry
- Potential Risk of Aluminum Leaching with Use of Certain Fluid Warmer Devices - Letter to Health Care Providers
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.