GORE TAG Thoracic Branch Endoprosthesis – P210032
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
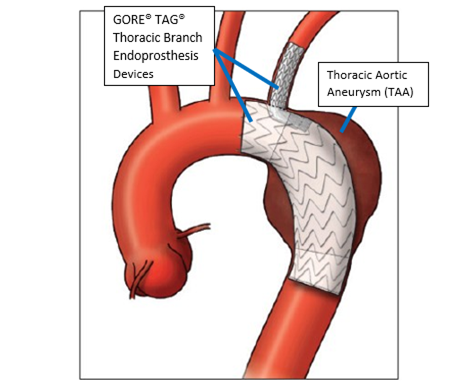
Product Name: GORE TAG Thoracic Branch Endoprosthesis (TBE)
PMA Applicant: W.L. GORE & Associates
Address: 3450 West Kiltie Lane, Flagstaff, AZ 86005 USA
Approval Date: May 13, 2022
Approval Letter: Approval order
What is it?
The GORE TAG Thoracic Branch Endoprosthesis is designed to repair the damage to the largest artery that is located in the chest (descending thoracic aorta). The Thoracic Branch Endoprosthesis consists of three implantable fabric tubes supported by a nitinol framework (stent grafts). Each stent graft has a catheter-based delivery system.
How does it work?
The GORE TAG Thoracic Branch Endoprosthesis is implanted in the descending thoracic aorta and into a blood vessel that branches off the aorta toward the head (left subclavian artery) to allow blood to keep flowing past the aorta’s damaged or diseased parts.
When is it used?
Doctors use the GORE TAG Thoracic Branch Endoprosthesis for endovascular repair of lesions of the descending thoracic aorta in patients who are at high risk for neck operation (surgical bypass). This device can repair several types of damage (lesions), including:
- Aneurysm, a diseased, weakened and bulging section of the aortic wall
- Transection, a rupture or tear of the aortic wall, typically from blunt force trauma
- Dissection, when the inside lining of the aorta tears away from the outer wall.
What will it accomplish?
In a clinical study evaluating the safety and effectiveness of the GORE TAG Thoracic Branch Endoprosthesis, 84.9% (62 out of 73) of patients with aneurysm, 87.5% (91 out of 104) of patients with dissection, and 100% (6 out of 6) of patients with traumatic transection achieved successful device implantation without device failure, aortic tears (rupture), lesion-related death, disabling stroke, permanent paralysis of part (paraparesis) or all (paraplegia) of the lower body, new onset kidney (renal) failure requiring dialysis, or the need for additional related procedures (reintervention) through the 12 month period.
When should it not be used?
The GORE TAG Thoracic Branch Endoprosthesis should not be used in patients who have:
- Known sensitivities or allergies to the device materials
- Conditions that might cause a graft infection
- Known hypersensitivity to heparin, including those patients who have had a previous incident of heparin-induced thrombocytopenia (HIT) type II
Additional information (including warnings, precautions, and adverse events):
- Summary of Safety and Effectiveness Data (SSED)
- Labeling (Instructions for use)
- Labeling Part 2 (Patient information)
- PMA Database Entry