Senza Spinal Cord Stimulation (SCS) System – P130022/S042
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Senza Spinal Cord Stimulation (SCS) System
PMA Applicant: Nevro Corporation
Address: 1800 Bridge Parkway Redwood City, CA 94065
Approval Date: January 18, 2022
Approval Letter: Approval order
What is it?
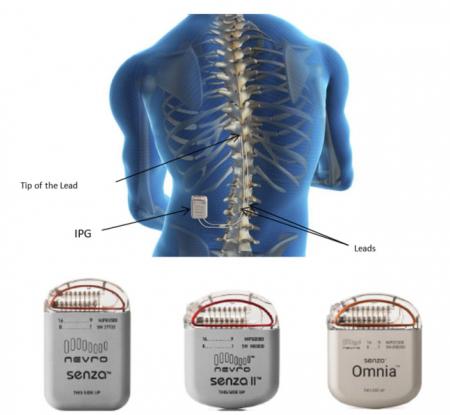
The Senza Spinal Cord Stimulation (SCS) system is an implanted, rechargeable spinal cord stimulation system intended to treat long-term (chronic) pain in the trunk or limbs that is difficult to manage (intractable).
The main components of the SCS system include an implanted signal generator that is connected to one or two implanted leads and a remote control that can turn the stimulator ON or OFF to allow adjustments of therapy settings.
This supplement expands the Senza SCS systems’ Indications for Use when programmed to a frequency of 10 kHz, to aid in the management of intractable back pain when the patient is not a candidate for back surgery, and back surgery hasn’t been performed previously.
How does it work?
The implanted signal generator receives radio signals from the remote control. The signals tell the signal generator when to deliver appropriate stimulation to the spinal cord. The external remote control is battery operated and can be controlled by the patient or a health care provider.
When is it used?
The SCS system when programmed to a frequency of 10 kHz, is used as an aid to manage chronic pain in the trunk or limbs, including one-sided or two-sided pain associated with failed back surgery syndrome, intractable low back pain, leg pain, pain associated with nerve damage caused by diabetes (diabetic neuropathy), and refractory back pain when the patient is not a candidate for back surgery and back surgery hasn’t been performed previously.
What will it accomplish?
The SCS system when programmed to a frequency of 10 kHz, may help treat chronic, intractable pain in a patient’s low back, trunk or limbs, when there is no prior back surgery and the patient is not a candidate for back surgery.
When should it not be used?
The SCS system should not be used in patients who:
- Cannot operate the SCS system
- Have not received effective pain relief during trial stimulation
- Are poor SCS surgical candidates