Testimony
Event Title
FDA User Fee Reauthorization: Ensuring Safe and Effective Medical Devices
March 31, 2022
- Testimony of
-
Jeffrey E. Shuren, MD, JD
Director - CDRH Offices: Office of the Center Director
INTRODUCTION
Chair Eshoo, Ranking Member Guthrie, and Members of the Subcommittee, thank you for having me here today. I'm Jeff Shuren, Director of the Center for Devices and Radiological Health (CDRH or the Center) at the Food and Drug Administration (FDA, the Agency, or our). Thank you for the opportunity to testify today on the reauthorization of the Medical Device User Fee Amendments (MDUFA) and our efforts to facilitate timely access to safe and effective medical devices for all Americans. We appreciate the efforts of Congress, and this Committee particularly, in successfully reauthorizing this program in previous cycles and look forward to continuing our partnership this year.
MDUFA
Enacted by Congress in 2002, MDUFA is a user fee program through which medical device
companies pay fees to FDA when they submit a request for marketing authorization, or certain other submissions, or register their establishments with FDA. The program includes commitments between the U.S. medical device industry and FDA to improve the predictability, transparency, and consistency of regulatory processes, which are intended to reduce the time for FDA to make a decision about whether to authorize marketing of a device. MDUFA has been reauthorized every five years since Congress first established the program in 2002. As the program has evolved, FDA and industry have successfully negotiated agreements to improve patient access to medical devices and streamline regulatory processes, all while assuring the safety and effectiveness of devices that patients and healthcare providers depend upon.
We have seen tremendous evolution and progress in FDA's medical devices program since inception of MDUFA. Prior to MDUFA enactment, FDA's devices program was in a far different place than the program we see today. We saw much longer review times, which led to less predictability and transparency for industry and patients. The investments made in the previous reauthorizations helped the MDUFA program make substantial progress. For example, we advanced more aggressive performance goals for 510(k) and premarket applications (PMA), including shared outcome goals; we added performance goals for Pre-Submissions (which provide an opportunity for a sponsor to obtain FDA's feedback prior to an intended submission such as an Investigational Device Exemption (IDE) or marketing application), and De Novo requests; as well as added process improvements for real world evidence, digital health, patient engagement, and use of consensus standards. As a result of these developments, we have seen an increasing number of innovators bring their devices to the U.S. first, before seeking to market them in other nations. We are seeing the pipeline of innovative new devices in the U.S. continues to become more robust, improving patient access to medical devices overall – with access being an important indicator of success for patients who may not have approved/cleared/marketed alternatives. It also demonstrates that a strong MDUFA agreement enables patients to have access to more innovative and better performing devices – and therefore more options – than at any other time in our history.
The draft MDFUA V reauthorization proposal was submitted to Congress on March 22, 2022. We expect to submit the final proposal following the close of public comments in April, and regret missing the statutory deadline to deliver the MDUFA V agreement this year. We take our obligation to provide the agreement to Congress in a timely manner very seriously, and know it is important for the Committees in both the House and Senate to have the opportunity to fully evaluate the agreement and engage with FDA and industry on the details because of how much is at stake, for FDA and our health care system. The deliberations on this agreement ran much longer than we intended, but it was critical that we took the time to deliberate and reach consensus on a strong, thoughtful agreement that assures the deviceprogram is appropriately resourced, and that we are supporting industry and innovators with a consistent, predictable, timely path to market for the safe and effective devices patients depend upon. We appreciate the patience of the Committee as we worked to reach an agreement that continues the progress made in the previous agreements towards advancement of medical device innovation, while maintaining FDA's standards. This is critical, as FDA and the device ecosystem face some of their greatest challenges. FDA's devices program continues to shoulder the unprecedented demands of the global COVID-19 pandemic, where the demand for medical devices has far exceeded anything we have seen in previous public health emergencies, while working hard to keep up with our MDUFA commitments as much as possible, and fulfill our ongoing mission of protecting public health and facilitating medical device innovation.
MDUFA IV
The MDUFA IV agreement enabled FDA to continue making progress on reducing review times and bringing devices to patients more quickly, while also enabling FDA to move forward in critical areas including advancing our work to support innovation in digital health, strengthening our partnership with patients, enhancing our program to adopt consensus standards, and improving our ability to leverage real world evidence towards regulatory decisions. In terms of review goals, we had a strong performance during the first half of MDUFA IV, continuing to meet and exceed performance goals, working to reduce the time for patients to have access to safe, new, innovative devices. In fiscal years 2018 and 2019, FDA achieved all of our submission review goals, met 21 of 24 performance enhancement goals, and FDA and industry met three of four shared outcome goals. Though not perfect, this performance evidence a continually robust pipeline for new and innovative devices, which is a positive condition for U.S. patients and our health care providers on the front lines. And with a robust pipeline comes an increase in workload, which reached some of its highest levels in key areas and substantially impacted our Center.
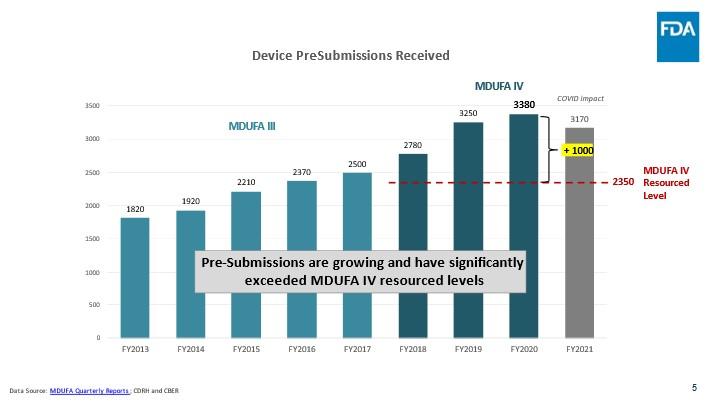
- Pre-Submission requests grew substantially beyond what was resourced in MDUFA IV. MDUFA IV assumed that Pre-Submission volume would hold steady at 2,350 submissions per year. In fact, FDA received over 3000 more Pre-Submissions than we were resourced to review in MDUFA IV, including more than 1000 submissions in FY 2020 alone. Growth in non-Breakthrough related Pre-Submissions was steady and linear for seven years prior to the pandemic (2013-2020), while growth in Breakthrough-related Pre-Submissions has been much more significant, increasing by an average of about 40 percent each of the last three years (FY 2019- FY 2021). With significant growth driven primarily by the popularity of the Breakthrough devices program, we expect to receive approximately twice as many Pre-Submissions per year by the end of MDUFA V than what was resourced in MDUFA IV.
- Submissions have and continue to become increasingly complex and, as a result, review of premarket submissions has become more resource-intensive. This rise in complexity is evidenced in several ways—
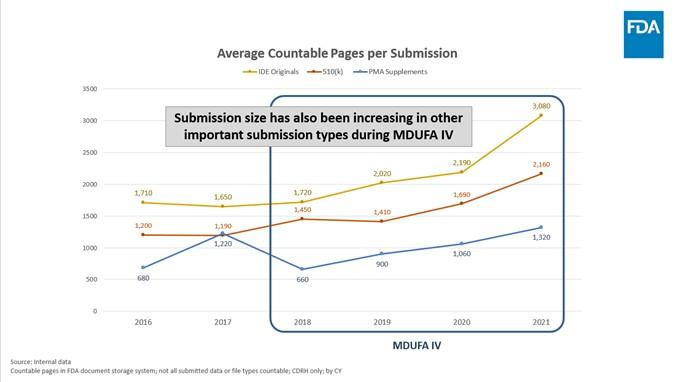
- Throughout MDUFA III, the average size of a 510(k) submission held steady, at around 1,000 pages per submission. In MDUFA IV however, the average size of a 510(k) has steadily and significantly increased, nearly doubling to an average of 2,000 pages per submission in 2021. This increase occurred while FDA's requests for industry to provide additional information decreased or remained stable. This increase in submission size is not just limited to 510(k)s. Average submission size has also increased in other important submission types during MDUFA IV. For example, the average size of an Original IDE grew by 1,300 pages (from around 1,700 pages per submission in 2018 to more than 3,000 pages in 2021) and the average size of a PMA Supplement doubled (from around 650 pages per submission in 2018 to more than 1,320 pages in 2021).
-
- FDA has approved or authorized record numbers of novel devices during MDUFA IV. In 2021, CDRH gave marketing authorization to 103 novel devices, an incredible achievement, especially during a time of increased demand on CDRH staff during the pandemic. Over the past decade, in fact, there were four times as many medical device approvals, authorizations, and clearances of novel technologies as a result of the innovative policies and approaches FDA has developed and implemented.
- Since FY 2018, FDA has granted more than 600 Breakthrough device designations – and more than 200 in the last fiscal year alone (FY 2021). The majority of sponsors of these products go on to submit multiple additional requests for FDA feedback (via Pre-Submissions) shortly after their designation is granted, with roughly 1/3 submitting five Pre-Submissions or more. More than 50 percent of companies receiving Breakthrough designations are either small or start-up companies (i.e., no or less than $1M in annual sales).
- FDA is also continuing to receive more premarket submissions overall than it has in previous years. For instance, in FY 2015, we received nearly 13,900 total premarket submissions, and in FY 2016 received nearly 14,300. In contrast, for FY 2021, FDA received over 16,400. This is approximately a 20 percent increase (or ~2,500 submissions) since FY 2015, and a 10 percent increase (or ~1,400 submissions) since the start of MDUFA IV.
We also note another challenge of MDUFA IV was the rising payroll costs in CDRH and the Center for Biologics Evaluation and Research (CBER), and the MDUFA inflation formula did not keep up. These payroll costs come from forces outside of the program's control—including government-wide, mandatory cost-of-living adjustments; automatic “step” increases for employees; and increased contributions to the federal retirement benefit. For CDRH, in FY 2022, the accumulated payroll cost impact of these factors for the MDUFA program is $45.5M. However, CDRH received $8.7M from the MDUFA payroll inflation adjustment, leaving a gap of $36.8M. These increased costs likewise placed additional strain on the devices program.
COVID-19 Pandemic
It is hard to overstate the impact the global pandemic has had on CDRH and the entire Agency, as it did for so many individuals, organizations, and communities around the world. Responding to this public health emergency (PHE) became central to our work and pushed us into a continuous all-hands-on-deck status, working oftentimes literally around the clock to facilitate the development and availability of pandemic-related devices as quickly and safely as possible. FDA's work to support access to devices for the COVID-19 response began in January 2020 – before the PHE was declared in the U.S. and two months before the pandemic was declared worldwide – due to the immediate need for COVID-19 tests and testing supplies, collection kits, personal protective equipment (PPE), ventilators, and other devices. To help combat the COVID-19 pandemic, FDA and CDRH staff have continued to go well beyond normal operating procedures to help ensure the availability of appropriately safe and effective COVID-19-related devices as quickly as possible.
From early in the pandemic, CDRH has actively reached out to and engaged other government agencies, medical device developers and international regulatory agencies, among other stakeholders. CDRH continues to hold weekly virtual town halls with industry to address COVID-19 test development and validation, as well as additional webinars and town halls addressing other policies and questions including PPE, 3D printed swabs and manufacturing disruptions during the public health emergency. CDRH staff have also interacted frequently with test developers and manufacturers through the Pre-Emergency Use Authorization (PEUA) process, including rolling reviews of information that helped to further expedite emergency use authorization (EUA) of critical medical devices for patients and health care professionals on the front lines. Since the beginning of the pandemic, CDRH has prioritized at-home tests, balancing speed with safety to ensure COVID-19 tests are appropriately accurate and reliable as supported by valid scientific evidence. CDRH has authorized 17 over-the-counter (OTC) at-home tests, resulting in hundreds of millions of additional OTC tests available monthly to American consumers. CDRH also took several additional steps, including: facilitating OTC COVID-19 test availability by issuing updated templates for EUA requests to streamline authorization of OTC tests; partnering with the National Institutes of Health (NIH) on the Independent Test Assessment Program (ITAP) to support FDA's evaluation of OTC COVID-19 tests that have the potential for manufacturing at significant scale, which resulted in two OTC authorizations of tests this year; and triaging our review efforts to focus on tests that ensure the biggest public health impact. We continue to grant EUA requests and take other actions, and we are proud that these contributions continue to help to facilitate the availability of critical devices and supplies for health care providers and patients.
We also saw innovators across the device ecosystem mount a remarkable response – medical device manufacturers large and small turning their production lines to different types of devices, and non-traditional manufacturers who came forward to manufacture devices for the first time – all to meet the needs of an unforgiving pandemic. Our team worked closely with them, night and day, to review EUA and Pre-EUA submissions, and the volume of EUA requests quickly surpassed (by several orders of magnitude) that of any prior PHE or emergency. It is important to appreciate that this enormous addition to our workload to review EUA and Pre-EUA submissions could not be supported by MDUFA funds. FDA engaged in an unprecedented effort to engage with sponsors from the outset, to provide regulatory flexibility where appropriate, and to handle the influx of EUA submissions along with a simultaneously increasing volume of MDUFA work. In doing so, FDA contended with a workload that far exceeded our capacity.
- FDA has received approximately 8,000 EUA and Pre-EUA requests for devices since January 2020 (including approximately 900 so far in fiscal year 2022), and continues to receive over 130 EUA and PEUA submissions a month
- To date, we have granted EUAs or traditional marketing authorizations to over 2,100 medical devices for COVID-19, including 15-times more EUAs for this PHE than all other previous PHEs combined. This includes ventilators and novel devices such as extracorporeal blood purification devices, as well as novel indications for devices such as continuous renal replacement therapy devices, for which FDA had not issued EUAs before. All in all, CDRH has reviewed and cleared over 1,300 510(k) devices for COVID-19 and future pandemics.
- We also issued 28 guidance documents (as well as 21 revisions) outlining policies to help expand the availability of medical devices needed in response to COVID-19.
- FDA also supported authorization and patient access to EUA devices and other devices through monitoring safety signals and medical device reports, publishing 23 letters to health care providers and 97 safety communications.
During 2020 and 2021, we also experienced an increase in conventional premarket submissions, as noted above. The enormous COVID-19-related workload taken together with the increase in our “regular” workload inevitably led to some delays and a backlog in the medical device review process.
FDA appreciates the impact this has had on companies across the country. This is why we have been transparent about the backlog of device submissions, issuing public communications and discussing expected impacts for sponsors during town halls, webinars, and in meetings with industry and other stakeholders. This is also why we have worked hard to reverse the backlog, for COVID-19 and non-COVID-19 devices, all while continuing to respond to the pandemic. Among other actions, we have adopted agile, interactive, and innovative approaches to review of EUA requests, published dozens of guidance documents and “EUA templates” to clarify agency recommendations and streamline review, implemented a front-end triage process to identify devices that would have the greatest impact on public health, reallocated our staff and resources from product areas less impacted by COVID-19 to those with increased submission volume, and made use of overtime. We greatly appreciate the support from Congress, particularly in the form of supplemental funding we used to leverage contractors and to hire temporary staff to help review EUAs.
MDUFA IV Performance
The strain from the pandemic, as well as a workload that exceeded assumptions made in the MDUFA IV agreement, resulted in failure to meet some of our MDUFA IV goals. Specifically, we fell short of our goals, or are likely to, in the following areas:
- For FY 2020, seven of 16 review goals are still pending, six were met, and three goals were missed. The missed goals include:
- The substantive interaction goals for:
- 180-day PMA supplements, and
- 510(k)s.
- The decision goal for Dual 510(k) and CLIA Waiver by Applications with no advisory committee input.
- The substantive interaction goals for:
- For FY 2021, seven of 16 review goals are still pending, three have met the goal, and six goals were missed. The missed goals include:
- The substantive interaction goal for:
- Original PMAs and panel-track supplements,
- PMA 180-Day supplements, and
- 510(k)s.
- The decision goals for:
- Original PMAs and panel-track supplements with no advisory committee input,
- 180-Day PMA supplements, and
- 510(k)s.
- The substantive interaction goal for:
FDA strives to meet all of our commitments, and we have built in additional transparency and accountability mechanisms in MDUFA V (which we will discuss in the next section). We also have statutory obligations to report to Congress on how we address and rectify missed performance goals. As we noted in the FY 2021 MDUFA annual performance report, FDA will continue to prioritize COVID-19-related work to address the ongoing public health need for safe and effective medical devices. As the COVID-19 pandemic continues to evolve, the volume of new EUA submissions for COVID-19-related products should begin to lessen in non-in vitro diagnostic (IVD) offices. This reduction will allow FDA to begin focusing review resources back to MDUFA-related activities, bringing review performance back to “pre-COVID-19” levels for non-IVD offices. FDA has already begun to reverse submission delays, and review times have improved significantly. Submissions for non-IVD products under review continue to generally meet MDUFA goals. The IVD Office is hiring, and will continue to hire, additional staff and contractors to address the increased volume of work in the office.
MDUFA V
MDUFA V supports both FDA's capacity to assess new medical device technologies and continues to provide a predictable, transparent path to market, while addressing critical resource gaps. The agreement strengthens our commitment to the foundation of the program – infusing more resources and people to review premarket device submissions. It also enhances accountability for the Center's performance and operations, and makes critical investments in the future of the program, to assure FDA has the resources to handle oversight and review of the robust pipeline of new technology and the innovations of tomorrow. It is an agreement that will ultimately lead to patients having timely access to new devices while upholding FDA's standards. Specifically, MDUFA V:
- Provides FDA with $1,783,931,700 over five years, helping assure the Center has resources it needs to handle a continually increasing workload resulting from strong innovation in the U.S., which impacted our Center before the COVID-19 pandemic.
- Supports improved performance across device types, to help assure U.S. patients have as rapid access as possible to innovative devices that are safe and effective.
- Increases accountability for the MDUFA program, to help assure critical transparency for industry, patients, and other stakeholders and helps assure FDA continues to meet its commitments under MDUFA V:
- Including an innovative new mechanism for add-on payments, unique to the MDUFA program, where approximately $115 million will be available for “add-on” funding during MDUFA V. If specified goals for 510(k)s, PMAs, De Novo requests, and Pre-Submissions are met in FY 2023-2025, FDA would apply additional user fees in FY 2025-2027 to support improvements in those goals.
- Providing for annual hiring targets for new positions, for the first time in MDUFA's history. If the target is missed by a specified percentage, a formula will be applied to calculate an offset of registration fees to be applied in the next annual fee setting cycle.
- Providing a cap for operating reserves in the carryover balance, which brings MDUFA into alignment with the other medical product user fee programs. If the carryover operating reserves grow beyond the prespecified level, additional funds will be sent back to industry in the form of offsets to registration fees.
- Retaining an independent contractor to conduct a MDUFA Workforce Data Assessment which would include:
- Assessing current methodologies and data and metrics available to represent MDUFA full time equivalent (FTE) resources (e.g., FTE burn and positions engaged in MDUFA process activities), including the subset funded by user fees, for each applicable Center and office; and
- Developing recommendations for improved methodologies and data and metrics to represent MDUFA FTE resources, including the subset funded by user fees.
- Providing additional transparency in the form of new reporting to industry and the public on use of MDUFA resources.
The agreement supports advancement of the patient perspective in regulatory decisions, continuation and expansion of the use of consensus standards to support device development and testing, leveraging of real-world evidence for regulatory decision-making, and enhanced coordination with international regulators, among other priorities.
MDUFA V pilots an innovative program to provide earlier, more frequent, and more strategic engagement with sponsors of products designated under the Breakthrough Devices Program and included in the Safer Technologies Program (STeP). The Total Product Lifecycle (TPLC) Advisory Program Pilot (TAP Pilot) will build upon lessons learned from these programs, as well as FDA's experience during the COVID-19 pandemic response, of engaging with sponsors through the pre-EUA process, which was critical for facilitating availability and accessibility of important products. The program will help to assure that device developers have a clear, predictable path to market such that patients have timely access to new devices. We believe it will help innovators avoid pitfalls in early product development, better ensure a clear, predictable path to market from development to bedside so that devices actually reach patients, and will continue to foster the innovation pipeline. The pilot will begin with a “soft launch” of up to 15 products in one CDRH Office of Health Technology (OHT) in FY 2023, and will expand to enroll up to 325 products across multiple OHTs by the end of MDUFA V. We will assess the pilot program and provide a public report on progress during MDUFA V.
Implementation of MDUFA V
As we look towards efficient and expeditious implementation of our new agreement, our Center will continue to face significant challenges after two long years of a global pandemic that continues to significantly impact our day-to-day work:
- FDA continues to receive a high volume of EUA and Pre-EUA requests for tests and other devices.
- An increasing number of EUA-authorized devices are being submitted for full marketing authorization. This includes 15 EUA-authorized devices that have already received full marketing authorization and an additional 16 under review.
- We continue to see an increase in submissions for devices that do not have EUAs, but are seeking marketing authorization for use during COVID-19 and future pandemics. These include various types of PPE, tests and testing supplies, needles and syringes, ventilators and respiratory assistive devices, dialysis equipment, and infusion pumps, among others.
We are committed to continuing the return to “normal operations,” but also know we will sustain some setbacks to our overall performance. This includes a continuing backlog of traditional device submissions for review. However, we have also begun to turn the corner – we have reduced the backlog of submissions by 44 percent. And even while in the middle of the pandemic, CDRH continued to authorize a record number of novel devices – over 100 each year – and we have been designating an increasing number of Breakthrough devices each year. Our Center has demonstrated time and time again that we do our best to meet and exceed our commitments; and, the fact there are more safe and effective medical devices on the market – more options for patients – than at any other time in U.S. history is a testament to these ongoing efforts. This makes all the difference for U.S. patients, and relies in part on the resources our program has to fulfill our mission. The MDUFA V agreement will be instrumental in getting the program fully back on track, allowing patients to continue to benefit from the robust innovation pipeline for medical devices in the U.S. We appreciate patience and support as we worked towards an agreement and, with the support of Congress and the MDUFA reauthorization, we can continue to accelerate access to new technologies that meet FDA's regulatory standards.
Thank you for the opportunity to testify today. I will be happy to answer your questions.