Testimony
Event Title
Next Steps: The Road Ahead for the COVID-19 Response
November 4, 2021
- Testimony of
-
Janet Woodcock, M.D.
- Before the
Introduction
Chair Murray, Ranking Member Burr, distinguished members of the Committee, I am Dr. Janet Woodcock, Acting Commissioner of the U.S. Food and Drug Administration (FDA or the Agency). Thank you for the opportunity to testify before you today to describe FDA’s coronavirus disease 2019 (COVID-19) response efforts. All of our efforts are in close coordination and collaboration with our partners, both within the Department of Health and Human Services (HHS) and across the Federal government, to help ensure the development, authorization, licensure, and availability of critical, safe, and effective medical products to address the COVID-19 public health emergency.
I want to note that this testimony is just a snapshot of some of our extensive work and is in the context of efforts across the Agency to address this pandemic. There are thousands of FDA employees who have been working on COVID-19 response efforts non-stop since the start of the pandemic. I want to commend and recognize their efforts and thank them for their dedication and service. I also want to thank all FDA employees who have continued to work on the myriad issues the Agency is responsible for that do not directly involve COVID-19.
From the beginning of this public health emergency, FDA has taken an active leadership role in the all-of-government response to the COVID-19 pandemic, inspired by the resiliency of the American people and our great innovators. FDA stood up an internal cross-agency group that continues to ensure we are doing everything possible to protect the American public, help ensure the safety, efficacy, and quality of FDA-regulated medical products, and provide the industries we regulate with the guidance and tools to do the same. We continue to focus on facilitating the development and availability of medical countermeasures to diagnose, treat, and prevent COVID-19, surveilling the medical product and food supply chains for potential shortages or disruptions, and helping to mitigate such impacts, as necessary to protect the public health.
Biologics, Including Vaccines
FDA’s Center for Biologics Evaluation and Research (CBER) continues to use every tool available to help facilitate the development and availability of vaccines and other biological products to combat the COVID-19 pandemic expeditiously and safely.
CBER is working on multiple fronts to address the COVID-19 pandemic, including:
- Helping to facilitate expedited clinical trials for vaccines and certain therapeutic biological products that hold promise to prevent or treat COVID-19 by providing timely interactions, scientific advice, and recommendations for individual sponsors and through guidance documents;
- Supporting product development and facilitating the scaling up of manufacturing capacity for high priority products to treat COVID-19 by conducting timely reviews;
- Expediting the review of Emergency Use Authorization (EUA) requests and Biologics License Applications (BLAs) for critical medical products to address COVID-19, including the evaluation of booster doses of COVID-19 vaccines and the use of COVID-19 vaccines in certain pediatric populations;
- Helping to ensure an adequate and safe blood supply; and
- Providing information to healthcare providers and researchers to help them submit expanded access investigational new drug application (IND) requests to permit the use of investigational products for patients with COVID-19.
Through our transparent scientific evaluation process, FDA has issued EUAs for three COVID-19 vaccines and has approved one vaccine for use in individuals 16 years of age and older. In doing so, we have relied upon the Agency’s rigorous standards for safety, effectiveness, and manufacturing quality. These COVID-19 vaccines were developed without cutting corners or compromising our regulatory and scientific standards. Intensive interactions between FDA and manufacturers minimized the time between different studies in the clinical development process; allowed seamless movement throughout the different phases of clinical trials; and simultaneously facilitated manufacturers proceeding with manufacturing scale-up before it was clear whether the safety and effectiveness data for a vaccine would support an EUA, allowing for quicker access to products once FDA reviewed the data and found the products met the Agency’s rigorous standards for authorization or approval.
For the approved vaccine, as well as those that have been authorized for emergency use, our process included a thorough evaluation of the data by the Agency’s career staff. We also solicited input from independent scientific and public health experts through our public advisory committee meetings for the COVID-19 vaccines that we have authorized. Throughout our scientific and regulatory process, FDA took additional steps to facilitate transparency, such as posting sponsor and FDA briefing documents and key decisional memoranda.
The COVID-19 vaccines that are available in the United States have shown clear and compelling efficacy in large, well-designed phase 3 trials. These vaccines are helping the country in the fight against this pandemic and have met FDA’s rigorous standards for safety and effectiveness to support either EUA or approval. All the COVID-19 vaccines that FDA has authorized for emergency use have far surpassed being at least 50 percent more effective than a placebo in preventing COVID-19, which was recommended in our June 2020 guidance document, Development and Licensure of Vaccines to Prevent COVID-19.1 A vaccine with at least 50 percent efficacy, we noted, would have a significant impact on disease, both at the individual and societal level.
As part of our continued efforts to be transparent and educate the public, we have a wealth of information on our website about the COVID-19 vaccines available for use in the United States. The information includes fact sheets for healthcare providers (vaccination providers) and vaccine recipients and caregivers, with important information such as dosing instructions; information about the benefits and risks of each vaccine; and topical Questions and Answers developed by FDA for the approved vaccine and each authorized vaccine.2
It is also important to highlight that, as part of each EUA or approval, manufacturers and vaccination providers are required to report serious adverse events, cases of Multisystem Inflammatory Syndrome (MIS), and cases of COVID-19 that result in hospitalization or death to the Vaccine Adverse Event Reporting System (VAERS), a national vaccine safety surveillance program jointly run by FDA and the Centers for Disease Control and Prevention (CDC).
COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of health problems following COVID-19 vaccination very seriously. FDA and CDC have implemented a coordinated and overlapping approach for continuous safety monitoring of all COVID-19 vaccines using state-of the art technologies. Specifically, the Agency’s monitoring following authorization of the COVID-19 vaccines uses a multi-pronged approach including: 1) passive surveillance using VAERS consisting of safety reports submitted by healthcare providers (providers in the CDC COVID-19 Vaccination Program are required to report adverse events following COVID-19 vaccination to VAERS), patients, parents and other members of the public, combined with 2) active surveillance, using large population-based healthcare datasets. These latter healthcare data systems offer a higher likelihood of detecting rare adverse events because they capture medical data on millions of Americans, cover diverse subpopulations (i.e., pregnant women, elderly, and patients with comorbidities) and can provide a longer duration of follow-up when compared to the prelicensure clinical studies. In addition, COVID-19 vaccine recipients are encouraged to enroll in CDC’s v-safe After Vaccination Health Checker smartphone-based tool that uses text messaging and web surveys to check-in with vaccine recipients over time after they receive a COVID-19 vaccine. Through v-safe, they can quickly tell CDC if they have any side effects after getting a COVID-19 vaccine. Together, the passive and active safety surveillance provide a coordinated and overlapping approach to vaccine safety monitoring for COVID-19 vaccines.
On August 23, 2021 FDA announced the first approval of a COVID-19 vaccine. The vaccine previously known as the Pfizer-BioNTech COVID-19 Vaccine was approved and is now marketed as Comirnaty, for the prevention of COVID-19 in individuals 16 years of age and older. Comirnaty has the same formulation as the originally authorized Pfizer-BioNTech COVID-19 Vaccine. Since the approval of Comirnaty, the Pfizer-BioNTech COVID-19 Vaccine has continued to be available under an EUA, for the two-dose primary series in individuals 12 through 15 years of age and as a third primary series dose for individuals 12 years of age and older who have been determined to have certain kinds of immunocompromised conditions. While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. To be clear, the American public should feel confident in receiving any of the available vaccines.
On September 22, 2021, FDA amended the EUA for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least six months after completion of the primary series in the following groups: individuals 65 years of age and older, individuals 18 through 64 years of age at high risk of severe COVID-19, and individuals 18 through 64 years of age whose frequent institutional or occupational exposure puts them at high risk of serious complications of COVID-19 including severe COVID-19.
On October 20, 2021 FDA further amended the EUA to clarify that a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine may also be administered at least 6 months after completion of the primary series to individuals 18 through 64 years of age with frequent institutional or occupational exposure to SARS-CoV-2.
On October 20, 2021, FDA also amended the Moderna COVID-19 Vaccine EUA to include use of a single booster dose at least 6 months after completion of the primary series in the following groups: individuals 65 years of age and older, and those 18-64 years of age at high-risk of severe COVID-19 or with frequent institutional or occupational exposure to SARS-CoV2. The Agency also amended the Janssen EUA to include the use of a single booster dose of the Janssen (Johnson and Johnson) COVID-19 Vaccine, administered at least 2 months after completion of the single-dose primary regimen to individuals 18 years of age and older. As of this announcement, all three COVID-19 vaccines have been authorized for a booster dose, but with varying eligibility between the mRNA and J&J vaccines.
Additionally, FDA authorized the use of heterologous, or “mix and match,” booster dosing in eligible individuals following completion of primary vaccination with a different available COVID-19 vaccine.
On October 29, 2021, the FDA authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include children 5 through 11 years of age. The authorization was based on the FDA’s thorough and transparent evaluation of the data that included input from independent advisory committee experts who overwhelmingly voted in favor of making the vaccine available to children in this age group. We are confident in the safety, effectiveness and manufacturing data behind this authorization. As part of our commitment to transparency around our decision-making, which included our public advisory committee meeting earlier last week, we have posted documents supporting our decision and additional information detailing our evaluation of the data will be posted soon. We hope this information gives parents the confidence they need to have their children vaccinated.
On the same day, FDA also authorized a manufacturing change for the vaccine to include a formulation that uses a different buffer; buffers help maintain a vaccine’s pH (a measure of how acidic or alkaline a solution is) and stability. This authorization is for two presentations: one for individuals 12 years of age and older and one for individuals 5 through 11 years of age. This new formulation is more stable at refrigerated temperatures for longer periods of time, permitting greater flexibility for vaccination providers. The new formulation of the vaccine developed by Pfizer Inc. contains Tris buffer, a commonly used buffer in a variety of other FDA-approved vaccines and other biologics, including products for use in children. The FDA evaluated manufacturing data to support the use of Pfizer-BioNTech COVID-19 Vaccine containing Tris buffer and concluded it does not present safety or effectiveness concerns.
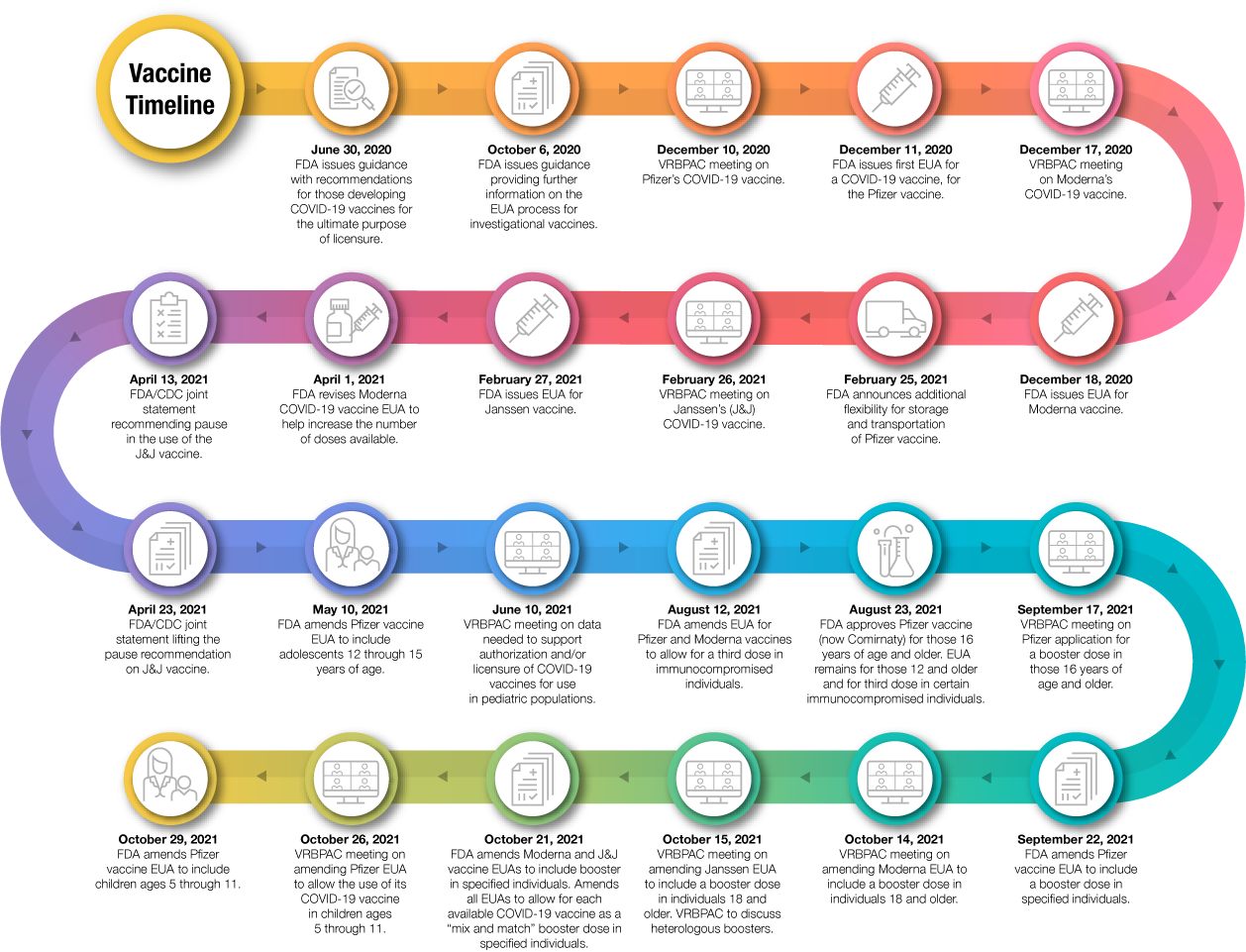
Figure 1
This pandemic is dynamic and evolving, with new data continuously emerging about vaccine safety and effectiveness. As we obtain more data about the safety and effectiveness of COVID-19 vaccines, including the use of a booster dose, we will continue to evaluate the rapidly changing science and keep the public informed.
At this time, data are not yet available to make a determination about how long either the approved or authorized vaccines will provide protection, and there is clinical uncertainty regarding whether the vaccines prevent transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from person to person, though it is clear they reduce the risk of severe illness. Additionally, although we do not yet know the full range of SARS-CoV-2 variants that each of the vaccines will protect against, there is evidence that the available vaccines protect against disease caused by variants circulating in the United States.
Finally, manufacturers whose COVID-19 vaccines have been authorized for emergency use are expected to continue their clinical trials in order to obtain additional safety and effectiveness information and pursue licensure (approval).
To date, having three authorized vaccines and one approved vaccine that meet FDA’s expectations for safety and effectiveness at this point of the COVID-19 pandemic is a tremendous achievement and a testament to the dedication of vaccine developers and FDA’s career scientists and physicians. We are highly engaged in ensuring that all COVID-19 vaccines meet the high quality that the American public expects and deserves. The Agency is very proud of these efforts, and we believe that the vaccines will help bring this pandemic to an end.
Therapeutics
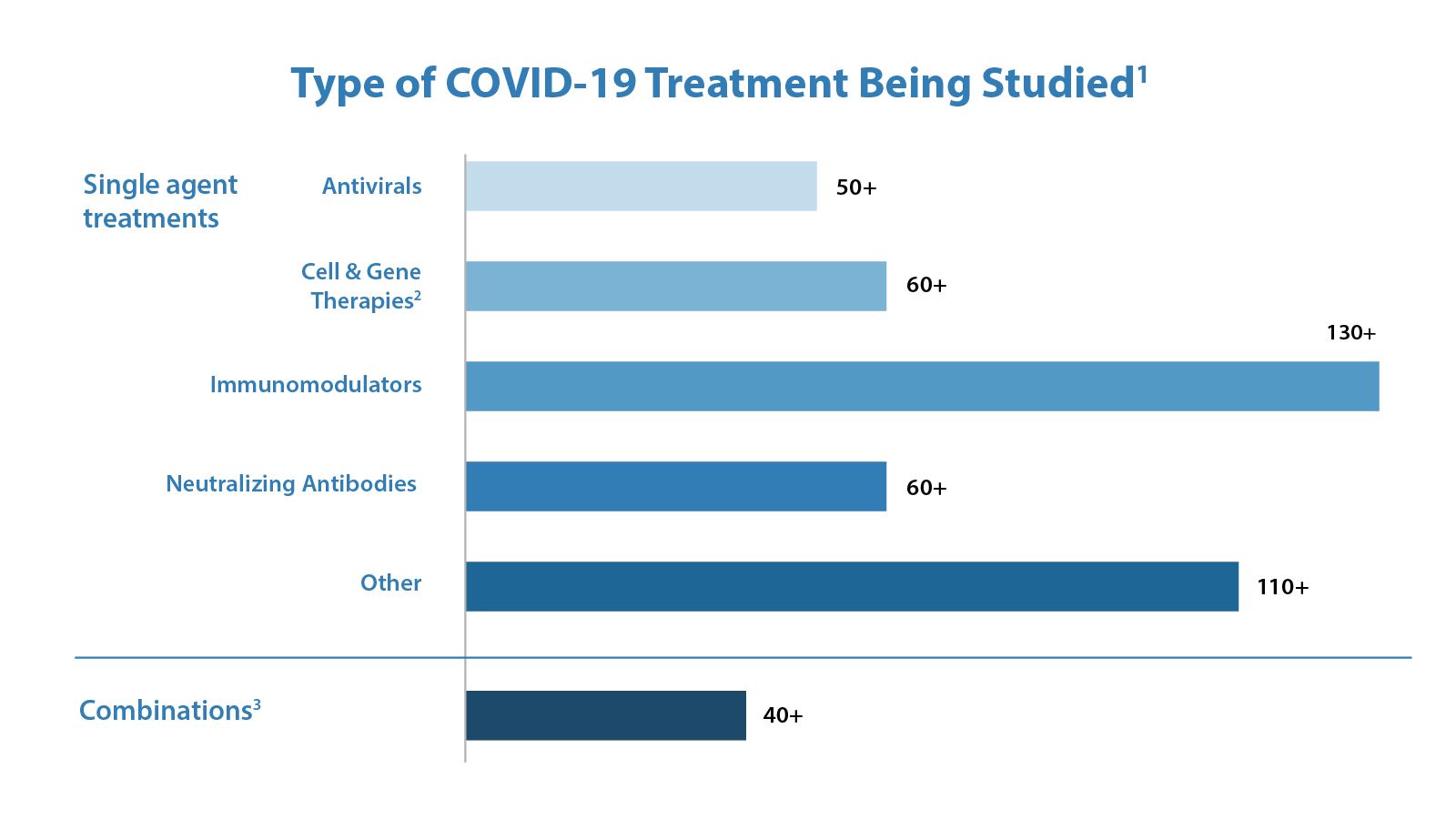
Since the beginning of the COVID-19 pandemic, FDA has been working tirelessly to facilitate the development and availability of therapeutics for use by patients, physicians, and health systems as expeditiously and safely as possible. FDA accelerated the development and publication of guidance and other information for industry and researchers on developing COVID-19-related treatments. Further, on March 31, 2020, FDA announced the creation of an emergency review and development program for possible therapies for COVID-19, the Coronavirus Treatment Acceleration Program, or “CTAP.” The primary goal of CTAP is to help accelerate the development of therapeutics for patients and consumers. The Agency has supported the program by reassigning staff and working continuously to review requests from companies, scientists, and doctors who are working to develop therapies. Under CTAP, FDA is using every available authority and regulatory flexibility to facilitate the development of safe and effective products to treat patients with COVID-19. As of September 30, 2021, there are more than 660 drug development programs in the planning stages and the Agency has reviewed more than 470 trials of potential therapies for COVID-19. These include antivirals, immunomodulators, neutralizing antibodies, cell and gene therapies, and combinations of these products. The diversity of therapeutic approaches being investigated is important because it rapidly expands our understanding of the effect of different categories of potential treatments.
Figures 2 & 3
1 Corresponds to number of safe to proceed INDs. Excludes INDs related to vaccines
2 For additional information, please see Cellular & Gene Therapy Products
3 Includes INDs with more than one product
FDA has approved one drug to treat COVID-19 and eleven therapeutics are currently authorized for emergency use. In considering EUA requests for therapeutics, we promptly and carefully evaluate the totality of the scientific evidence that is available on the product’s safety and effectiveness to determine, among other criteria, whether the product may be effective for its proposed authorized uses and whether the product’s known and potential benefits outweigh its risks. Our goal is to be as transparent as possible about the scientific basis for recommending that a drug or biological product be authorized for emergency use under section 564 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 360bbb-3) or for recommending that an EUA be revised or revoked. For example, last month, FDA announced an upcoming meeting of its Antimicrobial Drugs Advisory Committee (AMDAC) to discuss Merck and Ridgeback’s request for an emergency use authorization (EUA) for molnupiravir, an investigational antiviral drug to treat
COVID-19. The advisory committee will meet later this month to discuss the available data supporting the use of molnupiravir to treat mild-to-moderate COVID-19 in adults who have tested positive for COVID-19, and who are at high risk for progression to severe COVID-19, including hospitalization or death. The meeting was scheduled as soon as possible following the submission of the EUA request by the company. This timeline allows for the FDA to thoroughly evaluate the data and information submitted in the EUA request before the meeting and to be prepared for a robust public discussion with the advisory committee members.
FDA continues to work closely with manufacturers to mitigate and prevent shortages as the COVID-19 pandemic evolves. For example, the Agency has issued four EUAs to authorize the emergency use of certain therapeutic products intended to treat serious or life-threatening diseases or conditions (e.g., Acute Kidney Injury, Acute Respiratory Distress Syndrome) caused by COVID-19 after determining that sufficient FDA-approved alternatives to these products were not available to fully meet the emergency need. This has helped to alleviate shortages of some therapies that are essential for the care of critically ill COVID-19 patients. FDA is working with manufacturers to increase supplies to meet current demand by expediting review of applications. In addition, the Agency has prioritized the review of generic drug applications for potential treatments and supportive therapies for patients with COVID-19, such as antibiotics, sedatives used in ventilated patients, anticoagulants, and pulmonary medications. In June
2021, FDA reached a milestone of approving 1,000 original and supplemental generic drug applications since the start of the pandemic to help in the treatment of patients with COVID-19. This supports FDA’s everyday mission of improving access to safe, effective, high-quality treatment options, especially during the COVID-19 pandemic.
Medical Devices
FDA’s work to support access to medical devices for the COVID-19 pandemic began in January 2020 - before the Public Health Emergency (PHE) was declared in the U.S. and two months before the pandemic was declared worldwide - due to the immediate need for COVID-19 tests and testing supplies, collection kits, personal protective equipment (PPE), and other devices. The need for medical devices to respond to the COVID-19 pandemic has far exceeded what we experienced in any prior PHE. The first EUAs issued for the COVID-19 PHE were for medical devices, and the volume of EUA requests quickly surpassed (by two orders of magnitude) that of any prior PHE or other situation. Since January 2020, FDA has received over 7,000 EUA and Pre EUA(PEUA) submissions for devices. Further, the emergency use requests included submissions for devices that FDA’s Center for Devices and Radiological Health (CDRH) had never received EUA requests for during prior PHEs. This included ventilators and novel devices such as extracorporeal blood purification devices, as well as novel indications for devices such as continuous renal replacement therapy devices. Since the start of the pandemic, FDA has issued EUAs or granted marketing authorization to over 1,700 medical devices for COVID-19-related uses. In addition, FDA rigorously monitored safety signals and medical device reports, using the information to publish 22 letters to healthcare providers and 7 safety communications. FDA completed other pivotal work activities such as addressing supply chain shortages and counterfeit products related to COVID-19.
Diagnostic tests are the first line of defense in an outbreak, and FDA plays an important role to ensure these work through the EUA review. The EUA pathway expedites access to accurate diagnostic tests during emergencies, when information gaps and false results may adversely affect individual patient care and public health decision making. Through this pathway, molecular diagnostic tests are able to be developed, validated, authorized, and deployed within weeks rather than several months to over a year, as is typical for test development and traditional premarket submissions. The Agency employed its EUA authorities to facilitate availability of tests in six previous PHEs and carefully reviews tests because false test results can adversely impact the nation’s response. In PHEs, FDA is generally open to receiving and reviewing EUA requests for tests from any developer, including commercial kit manufacturers and laboratories.
FDA sought to facilitate COVID-19 test evaluation and authorization through the development and availability of templates. The templates provide recommendations for test validation and a fill in the blank form to streamline the paperwork and make it easier for developers to provide information in support of a request for an EUA. Since providing the first template in January 2020, FDA has been in daily contact with test developers to answer questions and help them through the EUA process. This has proved to be a helpful tool for many. FDA had as many as ten posted templates and continues to update, add, and remove templates as the science evolves and as necessary to support developers of COVID-19 tests. As of October 8, 2021, these templates have received over 510,725 hits from those visiting FDA’s website. FDA also supported test developers through establishment of a dedicated mailbox, 24-7 toll-free hotline that ran until July 2020, the posting of over 100 frequently asked questions on our website, and by hosting 72 weekly virtual town halls for test developers. The Agency has worked with over 1,000 test developers since January 2020.
The Agency prioritizes review of EUA requests for at-home rapid antigen tests and is actively engaging with test developers to increase their availability. The Agency first announced this prioritization in the Spring of 2020, during one of its weekly virtual Town Halls on COVID-19 tests, due to their potential impact on test accessibility and public health. To further encourage such test development, on July 29, 2020, FDA posted a template for at-home diagnostic tests. This template includes recommendations for validating over-the-counter (OTC) tests for screening asymptomatic individuals with performance expectations that are lower than for lab-based tests. The Agency adopted this position to increase test availability, recognizing that the benefits of increased availability of OTC tests outweigh the risks associated with less sensitive technologies, particularly for rapid antigen tests.
Throughout the pandemic, FDA has also monitored evolving circumstances and growing scientific knowledge and made adjustments when appropriate to help streamline and expedite the path to market for these and other tests as much as possible while assuring they are supported by sound science. In March 2021, FDA obtained results from an NIH-sponsored study that supported further streamlining of FDA’s at-home test recommendations. Based on these data, on March 16, 2021, FDA created a streamlined pathway for the Agency to authorize tests with at least 80% sensitivity in symptomatic individuals, with sensitivity falling in a range as low as 70% in certain circumstances, to offer their test for OTC serial screening without additional data collection. Multiple tests were authorized under this approach within weeks.
FDA authorized the first OTC at-home test authorization on December 15, 2020, and more recently, on October 4, 2021, the Agency authorized the ACON Laboratories Flowflex COVID-19 Home Test, which the Agency expects to significantly increase the availability of rapid, at-home tests; by the end of 2021, the manufacturer plans to produce more than 100 million tests per month, and this number will rise to 200 million per month by February 2022. This authorization added to the growing list of tests that can be used at home without a prescription in the U.S.
FDA further streamlined the regulatory pathway for manufacturers developing over-the-counter at-home tests on October 25, 2021, by providing for labeling updates to facilitate OTC single-use testing for symptomatic individuals for tests currently authorized only for serial testing. The developers of those tests will now be able to request authorization to add single-use testing for symptomatic individuals without submitting additional data. For example, right now when people go to a pharmacy to buy an over-the-counter test, they are sold in two-packs. This change would allow tests to be sold in singles, meaning more individual tests for sale potentially at a lower price.
Additionally, FDA authorized another over-the-counter rapid antigen test, granting an emergency use authorization to Celltrion Diatrust for its COVID-19 Home Ag Test for OTC single-use testing for symptomatic adults and OTC serial testing for all adults. This brings another easy-to-use rapid COVID-19 test to market, the tenth OTC test FDA has authorized. This means that when people go to their pharmacy or a store to buy a test they can take at home, they will soon have an additional option, increasing supply and making more tests available on shelves for people. In line with the new update to its regulatory pathway, FDA also reauthorized the Quidel QuickVue at-home test to add over-the-counter single-use testing for symptomatic adults and children.
As of October 22, 2021, FDA has authorized 15 EUA requests for 10 discrete rapid, at-home tests. Three of the 10 tests were authorized within one week. And as of October 12, 2021, FDA also has authorized 31 EUAs for rapid tests for use at point-of-care, including schools.
Going forward, FDA continues to take steps to increase access to reliable, accurate rapid antigen tests. This includes continuing to prioritize review of EUA requests for at-home diagnostic tests, and increasing staffing on the antigen test review team as resources permit. FDA is actively working to increase the pipeline of at-home tests by engaging with companies to obtain data that can be used to support their EUA, encouraging developers with authorized POC tests to add at-home test claims, continuing engagement with RADx and international regulators, and conducting targeted outreach to manufacturers of home tests in non-U.S. markets.
As part of these efforts, FDA is supporting NIH/NIBIB’s new Independent Test Assessment Program (ITAP) which will establish an accelerated pathway to support FDA evaluation of tests with potential for large-scale manufacturing. This program is an extension of the RADx program and will help identify manufacturers of high quality tests and encourage them to bring those tests to the U.S. market, increasing options for people and overall supply and potentially lowering costs. In this new program, NIH, FDA, and other CDC and HHS experts will assess and conduct studies on over-the-counter tests and work with companies to compile proper data, work towards the right benchmarks for performance, and support other needs that will help ensure they are providing the best submissions possible for FDA’s regulatory review. NIH will provide reliable, independent laboratory and clinical data to FDA for test manufacturers that can scale up quickly. If tests meet FDA’s performance and quality standards, FDA will use this information to grant emergency use authorization (EUA). In this new program, HHS will prioritize new over-the-counter test applications that have the potential for manufacturing at significant scale. The goal is to accelerate the availability of more high-quality, accurate and reliable over-the-counter tests to the public as quickly as possible.
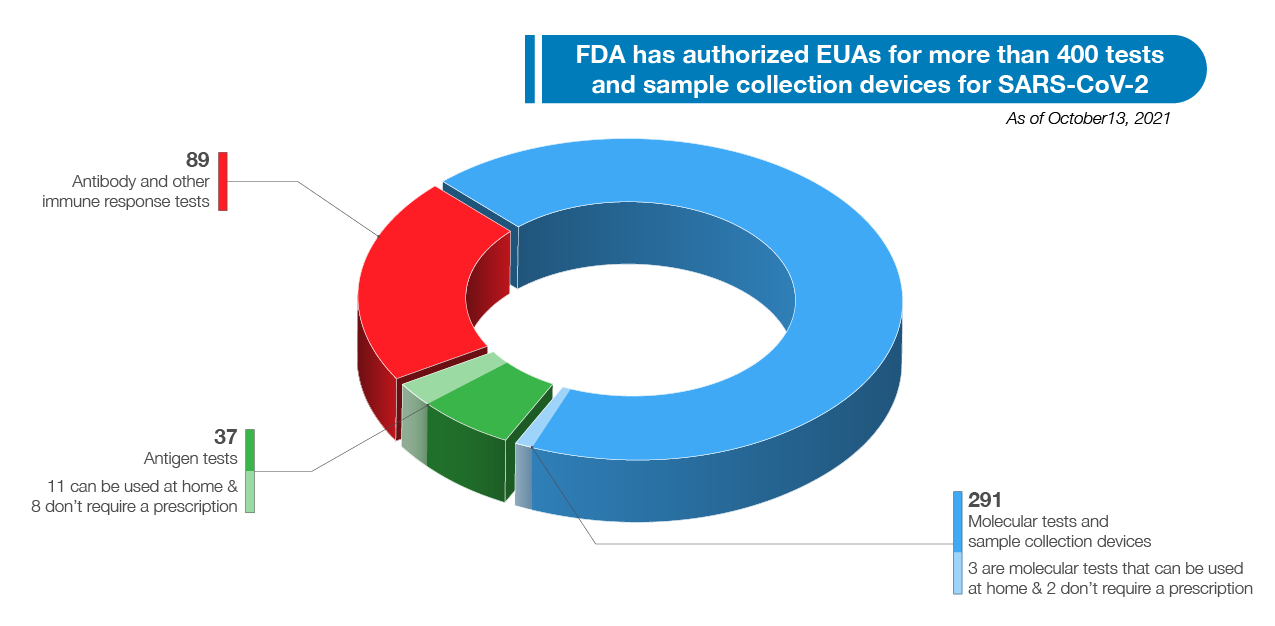
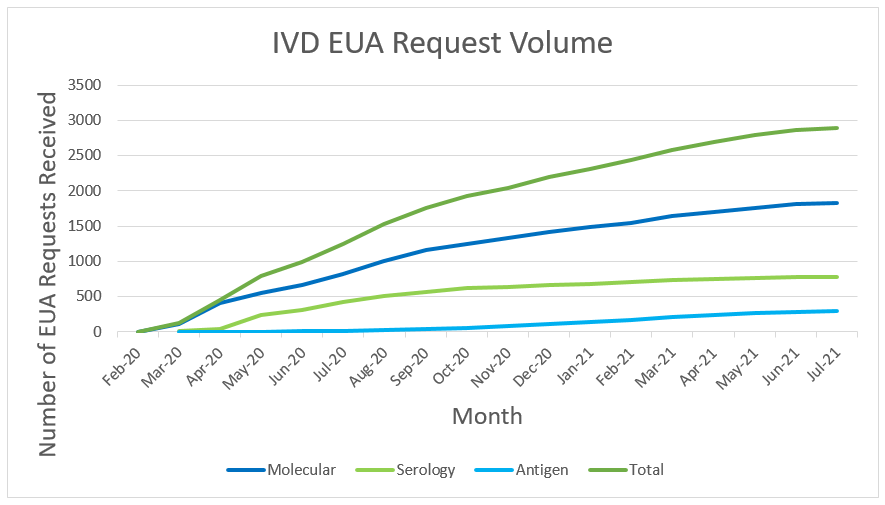
All of these actions will help put more tests on shelves when people go to buy an at-home test, unlocking more options and potentially lowering prices. FDA remains focused on facilitating a quick, efficient process to get good tests in the hands of all Americans such that the public can depend on their results. When the data support the test, we do not hesitate to authorize it. As of October 13, 2021, FDA has authorized EUA requests for over 400 tests and sample collection devices for SARS-CoV-2. As noted in Figure 5 below, these include 291 molecular tests and sample collection devices, 89 antibody and other immune response tests, and 37 antigen tests. Among these are 14 authorizations for diagnostic tests that can be run at home (three molecular and 11 antigen authorizations), ten of which do not require a prescription. We have also authorized 24 tests for serial screening programs (16 antigen and eight molecular). The volume and variety of available tests is a testament to FDA’s support of innovative test design and our commitment to public health. FDA will continue to take a flexible approach to COVID-19 tests to meet public health needs and increase access to testing for consumers, including at-home diagnostic tests, in an approach that is grounded in sound science.
Figure 4
Since early 2020, FDA has adopted agile, interactive, and innovative approaches to EUA review for all types of devices. For example, FDA developed the umbrella EUA approach to efficiently authorize multiple devices of the same type meeting the same criteria. The Agency has also issued 28 guidance documents (including 17 revisions) outlining policies to help expand the availability of medical devices needed in response to COVID-19. For example, developers of certain tests offered their tests, upon validation and notification to FDA, prior to issuance of an EUA during Agency review of the EUA request. Further, FDA made several improvements to our EUA review processes to make the most efficient use of our resources, including establishing a front-end triage process to identify devices that would have the greatest impact on the public health. These improvements incorporate the latest information on device availability and shortages, prioritizing novel or critical devices not yet available on the market or those that would address significant device shortages.
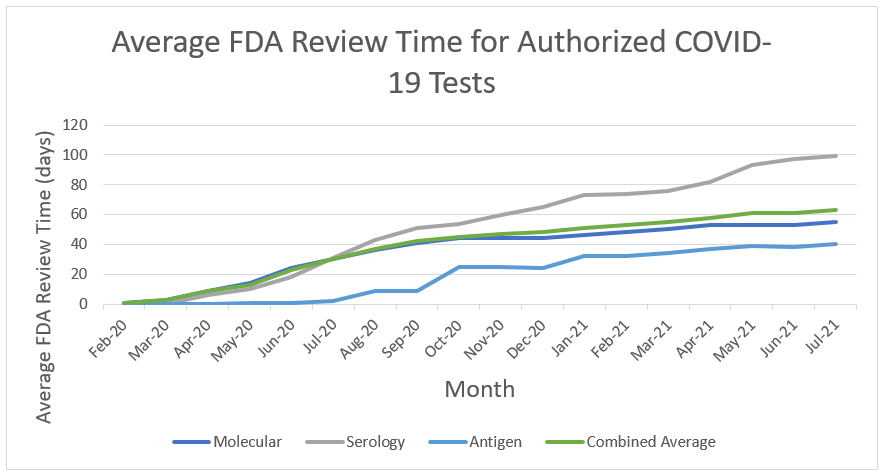
At the beginning of the pandemic, when there were relatively few diagnostic tests authorized, FDA’s priority was to rapidly increase the availability of tests. For medical devices, review times have increased over time as the number of EUA and Pre-Emergency Use Authorization (PEUA) submissions for medical devices have increased to unprecedented levels. This is demonstrated in the tables we have provided with review time for IVD EUA requests over time, and submission volume for IVD EUA requests over time (see Figures 6 &7 below). At the beginning of the pandemic, FDA was authorizing tests and other devices in as little as 1 or 2 days upon receipt of complete data packages. Congress has provided critical, one-time funding that the Center has used to hire term and temporary employees to help with the massive workload for tests, ventilators, PPE, and other products, but the workload has continued to greatly exceed capacity even with the additional support.
Figure 5
Figure 6
Please note that FDA has seen improvement in review times for IVD EUA requests as a result of the efforts noted above. As FDA’s workload increased, the time for review increased to up to 90 days for EUA requests received in September 2020. Review times for IVD EUA requests are now, on average, less than 40 days for those that were received in February 2021 and have been closed.
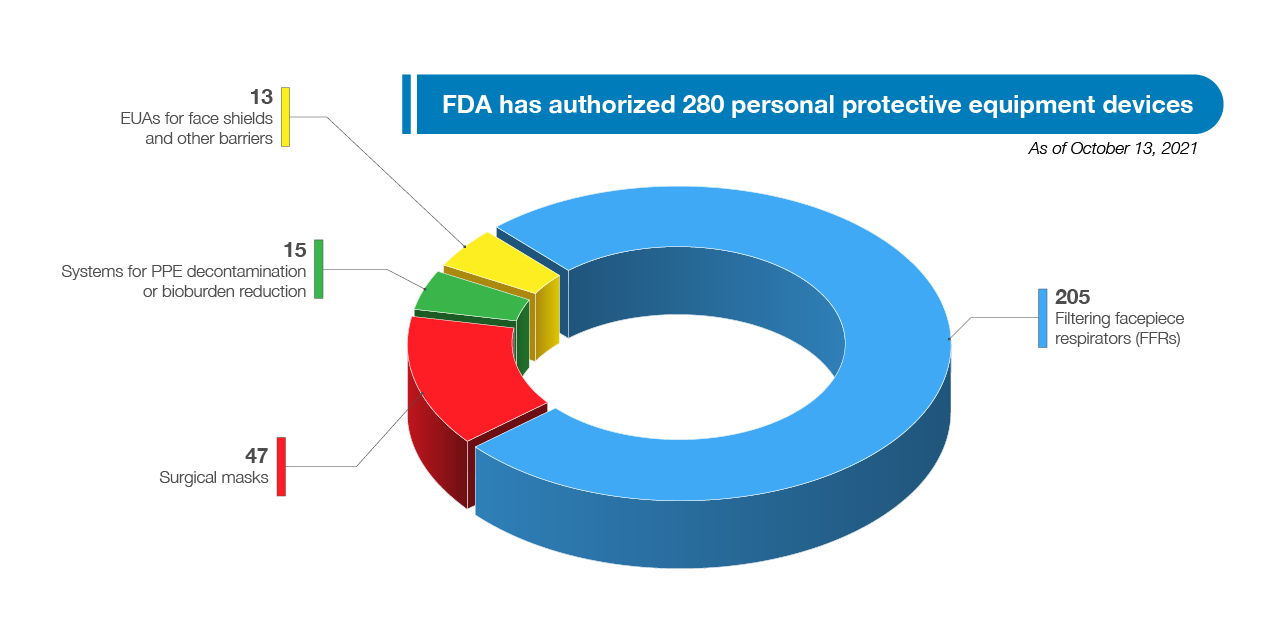
FDA has authorized a wide variety of other medical devices for use in combating the pandemic, including a wide range of PPE, ventilators, and other therapeutic devices. As of October 13, 2021, FDA has authorized 280 PPE devices including 47 surgical masks, and had authorized 205 filtering facepiece respirators (FFRs), 15 systems for PPE decontamination or bioburden reduction at the time there was a need for these types of devices due to PPE shortages, and 13 EUAs for face shields and other barriers intended to protect the user from bodily fluids, liquid splashes, or potentially infectious materials. See Figure 8. In addition to issuing EUAs, FDA has also cleared, through its premarket notification pathway, almost 400 PPE 510(k)s, which not only support the response to this pandemic but also future PHEs as well.
Figure 7
FDA recognizes that medical devices, particularly tests, will continue to play an important role in the next phase of the pandemic response. The Agency is continuing to monitor its policies, the marketplace, and national needs, and will continue to adapt as the circumstances of the evolving pandemic warrant.
Human and Animal Food
Throughout the pandemic, FDA has worked with Federal, state, and local partners, as well as industry, to help ensure a safe and adequate food supply for both people and animals.
While SARS-CoV-2 is not transmitted by food, some components of the food system are experiencing challenges and supply chain imbalances. We saw this at the outset of the pandemic with the dramatic shift in where people were eating, and most recently, we are seeing that the broad supply chain issues impacting so many commodities are also impacting food. Overall, food production and manufacturing in the U.S. has been remarkably resilient, but we continue to monitor the food supply chain systems closely to efficiently and promptly identify supply chain challenges and apply mitigation strategies when necessary.
In response to the pandemic, FDA’s Foods Program developed 21 Forward, a food supply chain data management tool, to help identify where risks for interruptions in the continuity of the food supply may be greatest. As part of 21 Forward, FDA continues to conduct targeted outreach to the food industry to offer additional resources and technical assistance in addressing challenges.
FDA also recognizes that food supply chain continuity and worker safety are two sides of the same coin. Thus, a robust food supply is dependent on the safety and health of the nation’s food and agricultural workforce. Along with our Federal, state, and local partners, we have provided best practices for food and agricultural workers, industry, and consumers on how to stay safe, and help ensure the continuity of operations in the food and agriculture critical infrastructure sector during the pandemic.
In collaboration with HHS, CDC, Health Resources and Services Administration (HRSA) and US Department of Agriculture (USDA), data from 21 Forward on the estimated numbers and distribution of food and agricultural workers have been made available to assist states with their vaccine distribution efforts for workers in the food and agriculture sectors, including migratory and seasonal agricultural workers. In addition, FDA has worked with its Federal partners to provide both COVID-19 and flu vaccination encouragement messages for the food industry.
FDA’s Coordinated Outbreak Response and Evaluation team has been working throughout the pandemic looking for signs of foodborne illness outbreaks and initiating responses as needed. FDA’s Center for Veterinary Medicine is monitoring the animal food supply and initiating needed foodborne illness and natural disaster responses. In terms of inspectional work, FDA investigators continue to conduct mission critical inspections domestically and abroad, including inspections and investigations in response to foodborne outbreaks, throughout the pandemic. FDA resumed standard operations for domestic surveillance inspections in July 2021. FDA continues to screen every line of every shipment of imported food entering the U.S. utilizing our Predictive Risk-Based Evaluation for Dynamic Import Compliance Targeting (PREDICT) tool. We adjusted the algorithm in PREDICT to place increased scrutiny on shipments from facilities where foreign inspections have been postponed. FDA has made greater use of our Foreign Supplier
Verification Program (FSVP) regulation to oversee compliance with FDA Food Safety Modernization Act (FSMA) requirements. The shift to remote FSVP inspections, along with other tools utilized by the foods program, has been critical to ensuring the safety of human and animal food from foreign suppliers during the COVID-19 pandemic. Since March 2020, FDA has conducted 2,362 FSVP inspections, which represents a 55% increase in inspections (1,527) over the 18 months prior to the pandemic. Additionally, FDA continues to identify human and animal foods that are unsafe, misbranded, or may cause a serious health concern for the public at the border with over 9,500 lines being refused admission since March 2020.
In July 2020, FDA announced the New Era of Smarter Food Safety Blueprint outlining the Agency’s plans over the next decade to create a more digital, traceable, and safer food system. The Agency has learned from its response to the pandemic that there is an accelerated need for certain goals in this blueprint, especially those involving supply chain continuity and resilience, modernized inspectional approaches, strengthening food safety infrastructures with regulatory partners, and the safety of foods ordered by consumers online. The number of consumers ordering food online has been steadily increasing over the years, but it has skyrocketed during the COVID-19 pandemic. FDA recently hosted a virtual Summit on E-Commerce to help the Agency improve its understanding of how human and animal foods are sold through e-commerce models and to identify courses of action for addressing potential food safety vulnerabilities, including those that may arise in the “last mile” of delivery.
Inspections, Compliance, and Protecting the Medical Supply Chain
Similar to their work protecting the food supply, import investigators have been on-site protecting the medical supply chain at our ports of entry, courier facilities, and the international mail facilities (IMFs) throughout the pandemic. Through continued vigilance, FDA has prevented unsafe and unproven pharmaceuticals and other medical products from entering the country. Since March 2020, with the cooperation of and in coordination with the U.S. Customs and Border Protection (CBP), FDA has received and destroyed almost 77,000 products, totaling over 13,932,173 capsules, tablets, and pieces of unapproved drugs.
Since March 2020, FDA has maintained the same level of screening for imported products as pre-pandemic and refused approximately 102,654 lines of imported, violative medical products. However, FDA has focused examinations on COVID-19 relief supplies to ensure compliant products are expedited while maintaining our commitment to refusing imported medical products that are unsafe, misbranded, unapproved, counterfeit, or may cause serious illness or injury to the public. In fact, our import investigators have evaluated donations of shipments destined for the Federal Emergency Management Agency (FEMA) and met the first vaccines (Pfizer Belgium) on their arrival into the United States in December 2020 to ensure proper transport, storage, and reconciliation of products, and also assisted with expediting the importation of other compliant vaccine-related shipments.
Despite generally pausing domestic and foreign surveillance inspections in March 2020 to safeguard the health and well-being of our staff, as well as employees at facilities we inspect, our investigators continued to conduct mission critical inspections both domestically and abroad to ensure FDA-regulated industries were meeting applicable FDA requirements. In July 2020, FDA resumed prioritized domestic inspections. To arm our investigators with the most reliable and accurate information, FDA developed a rating system to assist in determining when and where it was safest to conduct prioritized domestic inspections until we resumed standard inspectional operations in July 2021.
On May 5, 2021, FDA issued a report titled, “Resiliency Roadmap for FDA Inspectional Oversight,”3 outlining the Agency’s inspectional activities during the COVID-19 pandemic and its detailed plan to move toward a more consistent state of operations, including FDA’s priorities related to this work going forward.
The report described our oversight work during the pandemic and outlined the inspectional activities that the Agency had postponed due to travel restrictions or inability to ensure the safety of our workforce or the workforces within the industries the Agency regulates. The report also outlined the number of mission-critical inspections FDA completed during that time, such as inspections of facilities for which there was a drug shortage, inspections needed for the approval of novel drugs or drugs related to the potential treatment of COVID-19, support of pre-market and pre-license applications, and response to foodborne disease outbreaks or other food safety risks such as food contaminated with pathogens.
Additionally, the Resiliency Roadmap outlines FDA’s continued, successful use of alternative tools and approaches where inspections are not feasible, including remote regulatory assessments (e.g. requests to regulated establishments to remotely view records) and interactive evaluations that include remote livestreaming video of operations, teleconferences, or screen sharing, and leveraging information from trusted regulatory partners. For example, FDA made over 1,300 requests to human and animal drug and biological product manufacturers to remotely view records, to support on-time regulatory decision actions. In addition, since March 2020, FDA has added products from 23 firms to import alerts, based on records requests in advance or in lieu of inspection that FDA submitted pursuant to section 704(a)(4) of the FD&C Act.
Notably, FDA’s bioresearch monitoring program staff have conducted more than 130 remote regulatory assessments that were directly used in application decisions.4 The new tool was incentivized for and supported by industry and continues to provide the Agency with valuable information to assist with risk-based targeting for inspections. FDA recognizes that remote approaches do not replace inspections, and that there are situations where only an inspection is appropriate based on risk and history of compliance with FDA regulations.
The Resiliency Roadmap further outlined the ongoing steps the Agency is taking to resume standard operational levels of inspection activities, including how it intends to prioritize domestic and foreign inspections that were not performed during the pandemic. The plan highlighted a variety of possible scenarios given the continued uncertainty of the trajectory of the ongoing COVID-19 pandemic.
On July 1, 2021, FDA activated the base-case scenario and transitioned to standard operations for domestic surveillance inspections and other operational work through the end of September 2021. FDA has exceeded the goals for completing domestic surveillance inspections that were detailed in the Resiliency Roadmap and will be providing a more detailed accounting soon. We also exceeded our performance goal related to following up on previous inspections classified as official action indicated (OAI). Additionally, of the more than 13,500 applications for medical product approval or authorization received since March 2020, only 68 applications had been delayed due to the inability to conduct inspections — and a majority of these applications are not deemed mission-critical. Since the FDA issued the Resiliency Roadmap, the FDA has been able to make decisions on nearly half of the 68 applications.
When planning surveillance inspections, the Agency is prioritizing higher-risk establishments. This means that postponed inspections will be prioritized based on risk and conducted over a longer period of time, ultimately increasing the amount of time between inspections of certain lower-risk facilities in order to focus on products that present the greatest risk to public health.
The Agency launched a multi-year modernization effort in July 2021 to further transform our data enterprise platforms and cross-program interoperability infrastructure to better support innovation related to its regulatory oversight role. This includes adopting technology to support regulatory assessments to improve our remote receipt, review, and analysis of industry data and records, and improve remote interactions with industry entities to be easier, more efficient, more consistent, and more secure. This modernization effort includes a review of inspectional approaches using next-generation assessment technologies and improvements. FDA established an Agency-wide Inspectional Affairs Council (FIAC) that provides coordination of inspection approaches and assessment processes. The Agency intends to share more information on these efforts as this work progresses. FDA will continue to leverage and maximize every available tool and resource to meet its inspectional responsibilities, while achieving optimal public health outcomes.
Compliance and Enforcement
FDA exercises its regulatory authority by, among other things, issuing warning letters and by pursuing civil and criminal enforcement actions against firms and individuals who do not comply with regulatory requirements, including those distributing unapproved products with false or misleading claims that the products prevent, treat, mitigate, diagnose, or cure COVID-19. In March 2020, FDA launched Operation Quack Hack, which leverages the Agency’s expertise and advanced analytics to protect consumers from fraudulent medical products, including unproven cures, illegitimate test kits, and substandard or counterfeit respirators. FDA has sent hundreds of abuse complaints to domain name registrars and internet marketplaces. The Agency also has sent more than 260 warning letters to sellers of unproven products claiming to treat or cure COVID-19. Working with the Department of Justice (DOJ), FDA has sought and obtained preliminary injunctions that require defendants to halt the sale of unproven products claiming to treat or prevent COVID-19, including one product, “Miracle Mineral Solution,” that, when used as directed, is equivalent to industrial bleach. In addition, since the start of the COVID-19 pandemic, FDA has issued 17 warning letters to owners and/or operators of illicit internet pharmacy websites that offer for sale unapproved and misbranded drugs purported to treat COVID-19 to U.S. consumers.
In addition, the Office of Regulatory Affairs’ (ORA) Office of Criminal Investigations (OCI), working with other federal and local law enforcement agencies, has conducted criminal investigations involving unproven COVID-related products. In one such example, OCI investigated a physician who attempted to profit from the pandemic by marketing and selling an unproven COVID-19 treatment. The physician marketed and sold treatment kits—which included hydroxychloroquine—as a cure for COVID-19. In July 2021, the physician pleaded guilty to, among other things, trying to smuggle hydroxychloroquine into the United States to sell in his COVID-19 “treatment kits.” In another case, OCI investigated an individual who attempted to import approximately 1,000 unlawful COVID-19 test kits from China, which were intercepted at a FedEx facility in Memphis, Tennessee. As a result of OCI’s investigation, the individual pleaded guilty in October 2021 to a felony smuggling charge. OCI also has conducted criminal investigations to bring to justice those who tamper with COVID-19 vaccines. For example, OCI investigated a hospital pharmacist who tampered with COVID-19 vaccine doses at a Wisconsin hospital where he worked. On two successive nights, the pharmacist purposefully removed a box of COVID-19 vaccine vials from the hospital’s refrigeration unit intending to render the vaccines inert and no longer effective. Before the full extent of his conduct was discovered, 57 people received doses of the vaccine from these vials. In January 2021, the pharmacist pleaded guilty to two counts of attempting to tamper with consumer products with reckless disregard for the risk that another person will be placed in danger of death or bodily injury. He has been sentenced to three years imprisonment, followed by three years of supervised release, and he must pay approximately $83,800 in restitution to the hospital.
In addition, FDA investigators remain on the front lines at ports of entry, quickly examining, reviewing, and sampling import entries, and refusing admission of violative products where appropriate. We protect the supply chain in two equally critical ways: first, we help ensure safe products are coming in; and second, that illegal, dangerous, and fraudulent products do not get into the country. These efforts include partnering with CBP in establishing satellite laboratories at selected IMFs with scientists using state-of-the-art screening tools to rapidly identify unapproved, counterfeit and illicit products.
In March 2020, OCI, with the help of domestic law enforcement partners and foreign counterparts in the United Kingdom, led the investigation of fraudulent COVID-19 “treatment kits” that were falsely declared as “water treatment.” Import examination of these shipments found misbranded “kits” intended to treat SARS-CoV-2. As a result of this investigation, a British national was charged and arrested for shipping mislabeled and unapproved products. In May 2020, FDA worked with CBP to intercept several shipments of counterfeit facemasks, with the result that they were refused and destroyed before entering U.S. commerce.
More recently, FDA has taken steps to address hand sanitizer products that pose safety concerns, such as products that do not meet the required ethanol or isopropanol levels or that contain or may contain toxic ingredients like methanol or 1-propanol. Regarding the latter, substantial methanol exposure can result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death. Ingesting 1-propanol can cause central nervous system depression, which can result in death. FDA has tested several hundred products using field-based and laboratory-based tools and found more than a hundred violative products. The Agency warned consumers not to use contaminated hand sanitizers. FDA also has taken steps to help ensure that these dangerous or subpotent products do not enter domestic commerce, including coordinating with CBP to identify such products, and we have listed products made by more than 65 manufacturers on import alert. FDA also placed all alcohol-based hand sanitizers from Mexico on a countrywide import alert to help stop products from entering the U.S. that appear to be in violation until the Agency is able to review the products. That action marked the first time the FDA has issued a countrywide import alert for any category of drug product.
Medical Product Supply Chain
FDA monitors and responds to worldwide demand and supply chain disruptions for medical products caused by the COVID-19 pandemic.5 We work closely with manufacturers to help ensure they continue to notify the Agency of any permanent discontinuance or interruption of drug (human and animal), biological product, and device manufacturing in a timely manner and, as noted in FDA’s Fiscal Year (FY) 2022 budget, we are working to better position the Agency and our health care system to assure a strong domestic supply chain in future emergencies.6
This is especially important as the COVID-19 pandemic has exposed major vulnerabilities in the domestic supply chain that FDA continues to face as it works to help ensure access to the treatments and devices that patients and healthcare providers need.
In addition to our usual communications with drug manufacturers, we work closely with healthcare and pharmacy systems, hospitals, providers, and others on the frontlines of COVID-19 patient care to identify problems with access to critical care drugs used to treat COVID-19.
FDA understands the significant impact shortages can have on patient care and we are using our authorities to help prevent and alleviate disruptions. When we identify a shortage, we react swiftly to mitigate the impact to U.S. patients and health care professionals, and quickly share that information with the public. Restoring and increasing the supply of approved drugs has been the agency’s priority. In addition, where necessary, FDA has issued temporary policies during the COVID-19 emergency to respond to reports from hospitals of increased demand and interruptions in supply which have not resulted in a drug shortage but caused concern about continuing access to drugs to support hospitalized patients with COVID-19. We issued temporary policies for outsourcing facilities registered with FDA and pharmacists in state-licensed pharmacies or federal facilities, regarding the compounding of certain FDA-approved drugs used for hospitalized patients with COVID-19. The Agency has published guidance to help applicants and manufacturers provide FDA with timely and informative notifications about changes in the production of certain drugs, including animal drugs, and human biological products. We urged the timely submission of these notifications, which may assist in our efforts to prevent or mitigate shortages of such products. In addition, section 503B(a)(2)(A) of the FD&C Act permits outsourcing facilities to use bulk drug substances to compound drug products that appear on the drug shortage list in effect under section 506E of the FD&C Act at the time of compounding, distribution, and dispensing, when all conditions of section 503B are met.
Our experience with COVID-19 demonstrates that a strong domestic supply chain depends on a resilient supply chain for medical devices as well. Indeed, multiple entities – across both the public and private sector – collectively have important roles to play in strengthening the domestic medical device supply chain. FDA can play a critical role in identifying and preventing shortages for devices, because the Agency not only reviews and authorizes these products, but has unique, collaborative relationships that allow direct engagement with device manufacturers, patients, distributors, healthcare organizations, and other stakeholders. Even before the pandemic hit the U.S., there were disruptions in the supply chain due to higher demand for devices in other nations where COVID-19 was already prevalent and shutdowns in locations from which supplies were sourced. As a result, FDA began shortage mitigation activities for medical devices in January 2020 before the PHE was declared in the U.S., and two months before a pandemic was declared worldwide. The Agency took several actions to rapidly respond to supply chain needs, including reassigning 130 staff to perform shortages work across CDRH and contacting over 1,000 manufacturing facilities in 12 countries in just a few weeks’ time to get as much information as possible about critical devices.
In addition, FDA has conducted horizon scanning to assess demand for devices needed to respond to the pandemic, including PPE, ventilators, diagnostic supplies, infusion pumps, and non-contact infrared thermometers; and established a rapid response team, working with field personnel to address fraudulent imports. The Agency has likewise worked to prevent and mitigate shortages of testing supplies. For example, FDA collaborated with U.S. Cotton, one of the world’s largest manufacturers of cotton swabs, to develop and produce a polyester-based Q-tip-type swab for testing. FDA also collaborated with laboratories and clinical investigators validating potential alternative sources of control materials, transport media, and swabs. As individual developers validated these alternative components, FDA requested their permission to share their findings publicly so that others could benefit, and we posted these alternatives on our website. In this way, FDA has been serving as a clearinghouse for scientific information that the entire community can leverage to mitigate shortages and increase testing capacity. FDA continues to post this information on a rolling basis on an FAQ website so that labs have access to the latest information regarding alternative controls, transport media, extraction, instruments, and swabs.
Medical device shortages not only put patients in harm’s way but also jeopardize our health care workers on the front lines, during PHEs like the COVID-19 pandemic and every day in our health care system. Moreover, device shortages disproportionately affect at-risk populations and exacerbate health disparities. For these reasons, FDA continues to do all it can within its current authorities and resources to mitigate shortages and supply chain interruptions for COVID-19 and within the U.S. health care system generally.
Congress has acknowledged the importance of FDA’s work on shortages in our health care system and we want to continue working with this Committee and others to make sure FDA has the resources and authorities needed to ensure U.S. patients and health care providers have the products they need each day, and especially during public health emergencies.
Conclusion
FDA continues to advance its mission to protect and promote public health by helping to ensure the safety of human and animal food, and the safety and effectiveness of medical products. We take our public health mandate very seriously and will continue to work each day to help end this pandemic. We continue to communicate with the American public and make regulatory decisions based on data and sound science. I look forward to continuing to work with the Committee on these efforts and thank you again for the opportunity to testify today.
- 1. https://www.fda.gov/media/139638/download
- 2. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-frequently-asked-questions
- 3. https://www.fda.gov/media/148197/download
- 4. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/remote-interactive-evaluations-drug-manufacturing-and-bioresearch-monitoring-facilities-during-covid
- 5. https://www.whitehouse.gov/wp-content/uploads/2021/06/100-day-supply-chain-review-report.pdf
- 6. FDA Fiscal Year 2022 Justification of Estimates for Appropriations Committees (https://www.fda.gov/media/149616/download)