Expanded Access (Compassionate Use) Submission Data
CDER, CBER and CDRH Expanded Access INDs and Protocols (2016-2020)
On this page you will find:
- The Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) expanded access submission receipt reports for INDs and Protocols from 2016-2020
- The Center for Devices and Radiological Health (CDRH) expanded access requests from 2016-2020
The reports are broken down to the following:
FY 2016 – 2020 (most recent 5 years) Graphs of Expanded Access Submissions
To view the graph select the appropriate link below.
For older graphs, see archive page.
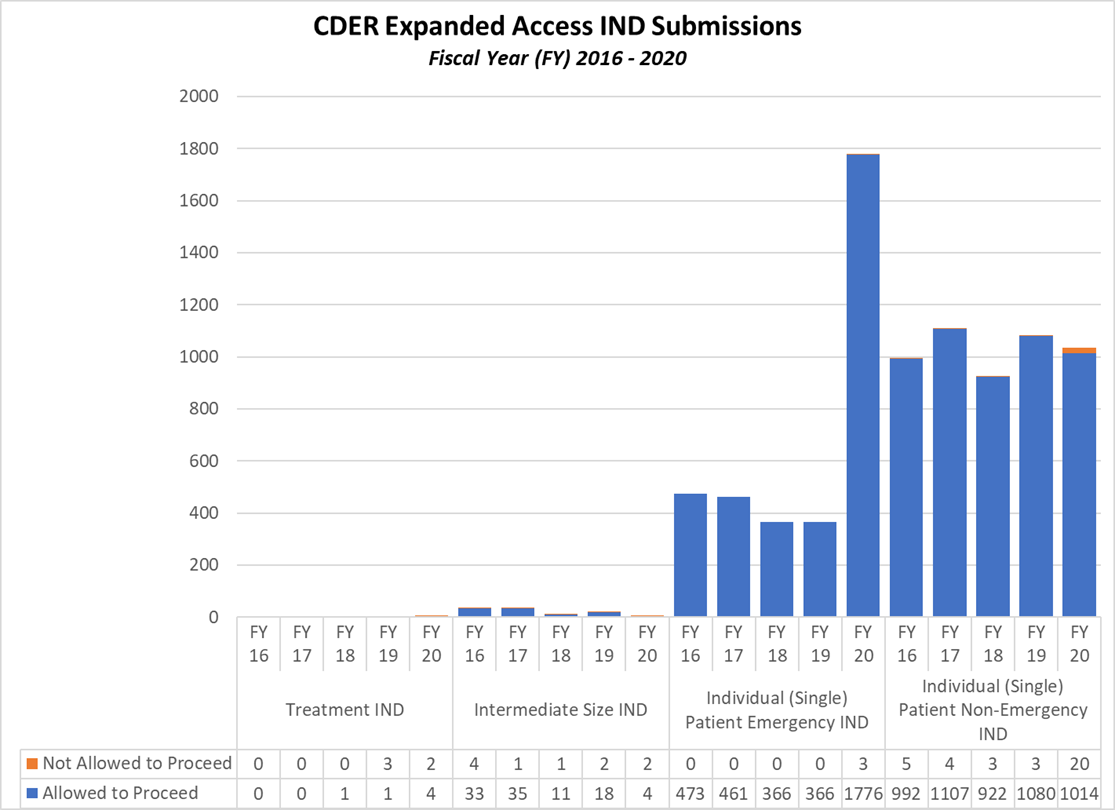
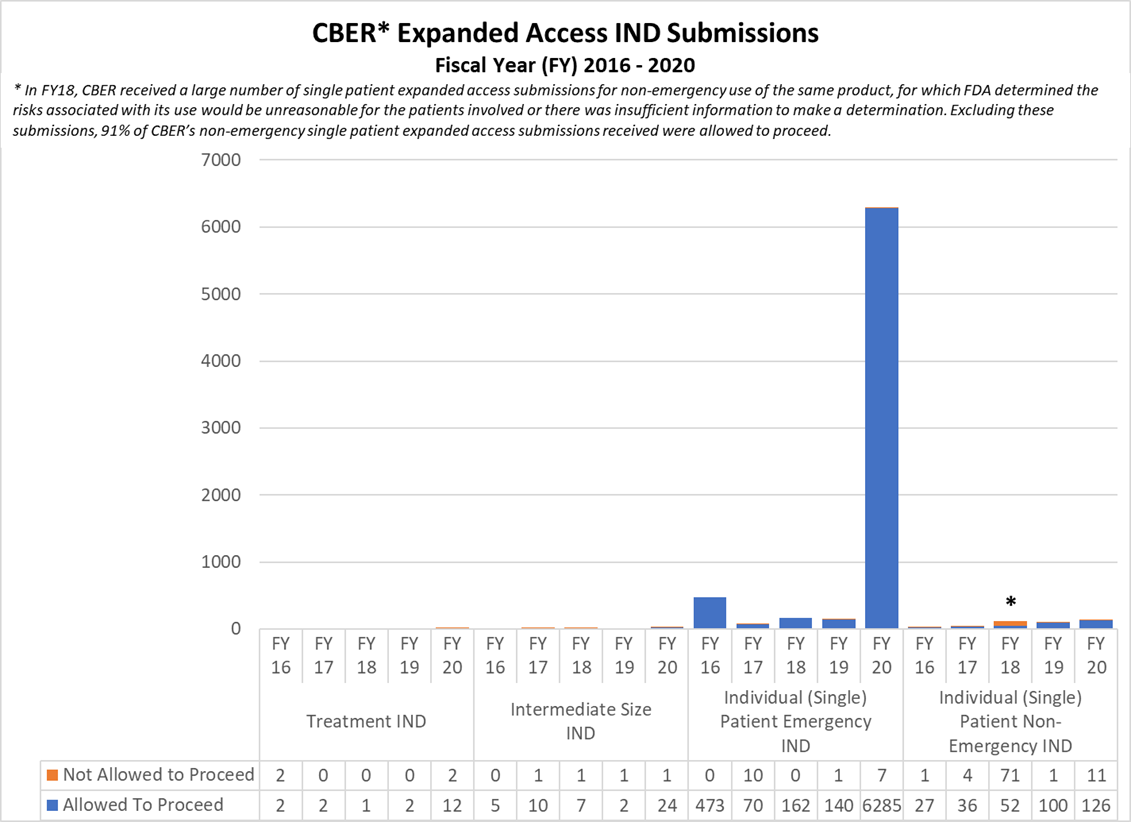
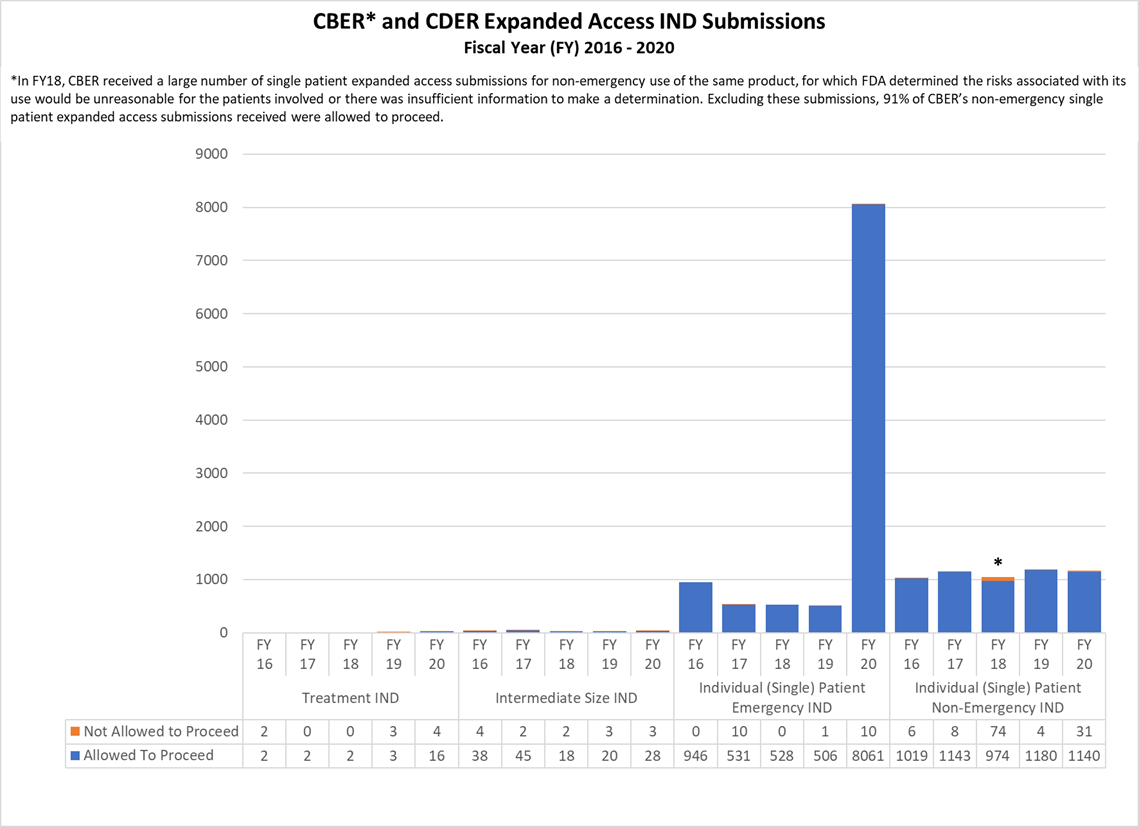
Expanded Access INDs for CDER and CBER (2016-2020)
* In FY18, CBER received a large number of single patient expanded access submissions for non-emergency use of the same product, for which FDA determined the risks associated with its use would be unreasonable for the patients involved or there was insufficient information to make a determination. Excluding these submissions, 91% of CBER’s non-emergency single patient expanded access submissions received were allowed to proceed.
For tables with older data, see archive page.
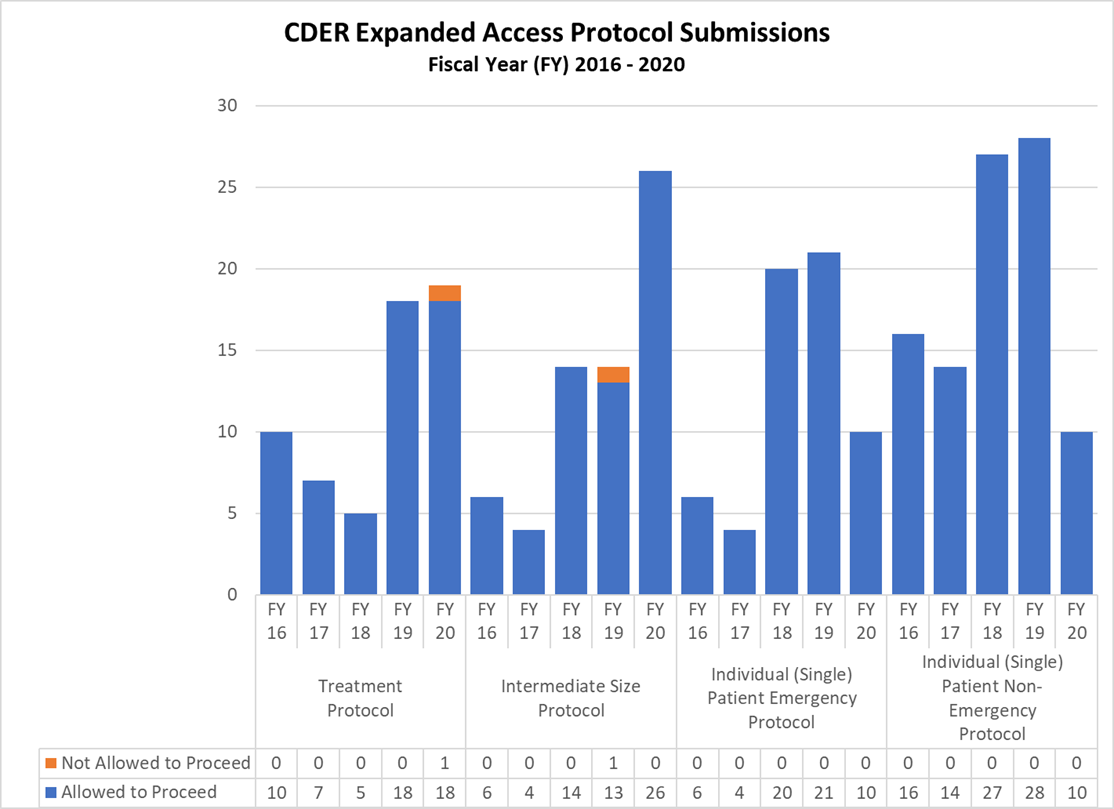
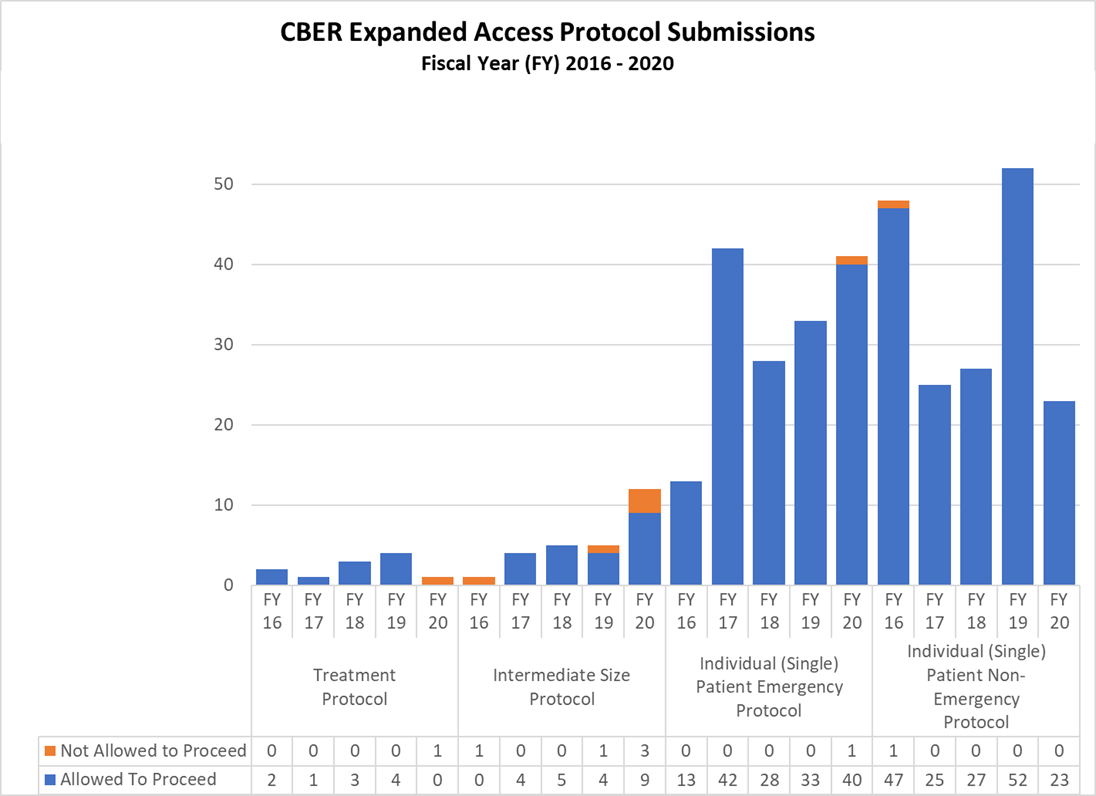
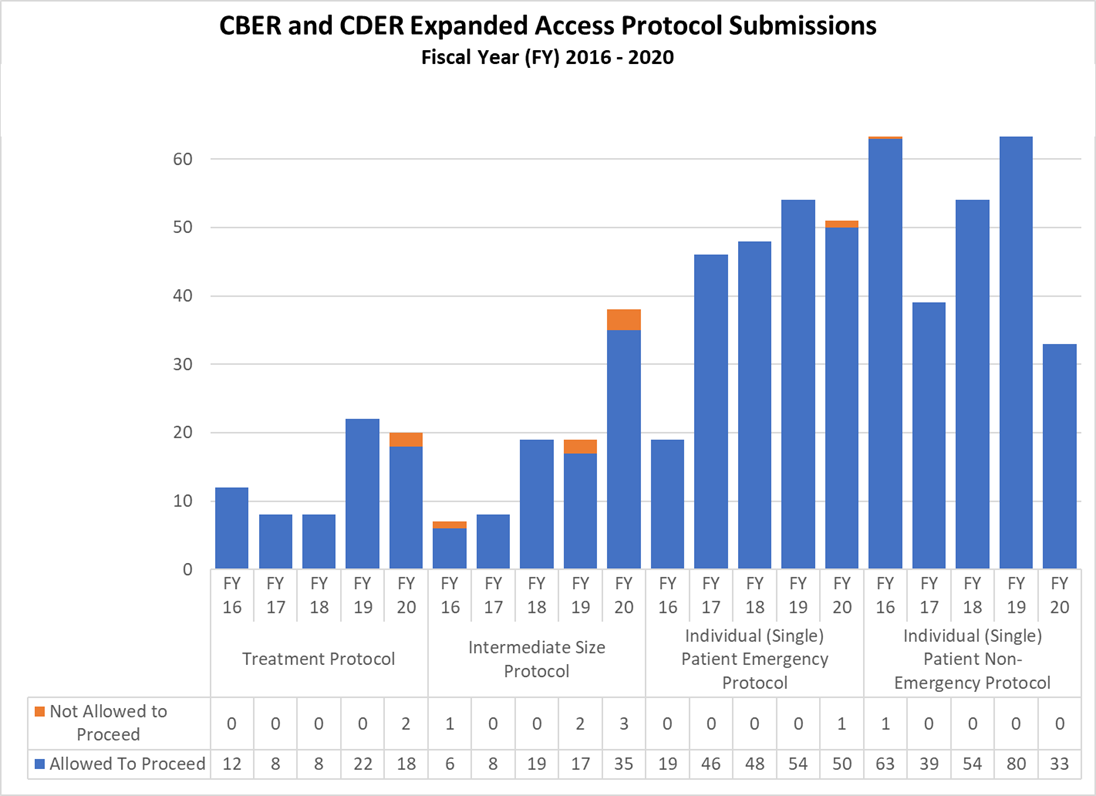
Expanded Access Protocols for CDER and CBER (2016-2020)
FY 2016 – 2020 Graphs of Expanded Access Submissions
CDER Expanded Access INDs and Protocol (2016-2020)
CBER Expanded Access INDs and Protocols (2016-2020)
Combined CDER and CBER Expanded Access Submissions and Protocols (2016-2020)
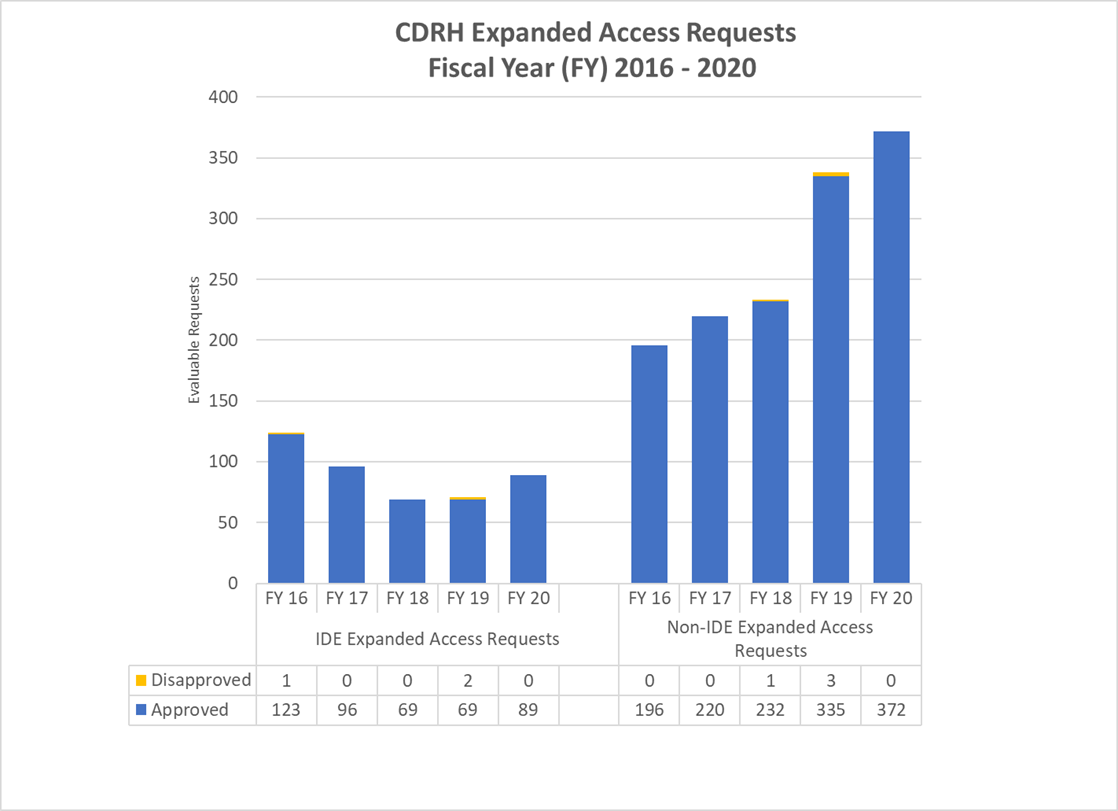
CDRH Expanded Access Requests (2016-2020)
FY 2016-2020 Graph of CDRH Expanded Access Requests
CDRH IDE Expanded Access Requests (2016-2020)
| Expanded Access Totals | |||||
|---|---|---|---|---|---|
| FY 2016 | FY 2017 | FY 2018 | FY 2019 | FY 2020 | |
| Requested Received | 136 | 100 | 79 | 76 | 92 |
| Evaluable Requests | 124 | 96 | 69 | 69 | 89 |
| Approved | 123 | 96 | 69 | 67 | 89 |
| Percentage Approved | 99.2% | 100.0% | 100.0% | 97.1% | 100% |
CDRH Non-IDE Expanded Access Requests(2016-2020)1
| Expanded Access Totals | |||||
|---|---|---|---|---|---|
| FY 2016 | FY 2017 | FY 2018 | FY 2019 | FY 2020 | |
| Requested Received | 198 | 230 | 257 | 352 | 384 |
| Evaluable Requests2 | 196 | 220 | 233 | 335 | 372 |
| Approved | 196 | 220 | 232 | 332 | 372 |
| Percentage Approved3 | 100.0% | 100.0% | 99.6% | 99.1% | 100% |
1 Expanded Access requests outside of an IDE are limited to single patient compassionate use requests.
2 Evaluable requests are requests in which a substantive review was performed on an original, non-IDE compassionate use request resulting in an approval or disapproval decision.
3 Based on approved requests to total number of evaluable requests.