Infant Formula Information and Ongoing FDA Efforts to Increase Supply
On Feb. 17, 2022, the FDA warned consumers not to use certain powdered infant formula products from Abbott Nutrition’s Sturgis, Michigan infant formula production facility. Abbott voluntarily ceased production at the facility as well as initiated a voluntary recall of certain products. We know the recall and facility shutdown have created hardships obtaining infant formula, particularly given the overall strains on supply chains experienced during the COVID-19 pandemic. As you consider options, talk with a health care provider for recommendations on changing feeding practices.
The FDA continues to work around the clock with our government partners and industry to ensure there's adequate infant formula available wherever and whenever parents and caregivers need it. More infant formula will be available in the weeks and months ahead.
How the FDA is working to increase supply
- Working with current infant formula producers that service the U.S. market to increase production. This includes working with the U.S. Department of Health and Human Services to use the Defense Production Act to ensure ingredients are available to sustain this production.
- Exercising flexibility by allowing the import of certain infant formula products from abroad that will result in millions of cans of infant formula being available on U.S. store shelves beginning in June 2022.
- Working closely with U.S. partners and domestic and international manufacturers to immediately increase the availability of specialized medical infant formula available in the U.S.
- Working with Abbott Nutrition under a consent decree to ensure Abbott Nutrition’s Sturgis, Michigan facility resumes production in a safe and sanitary manner.
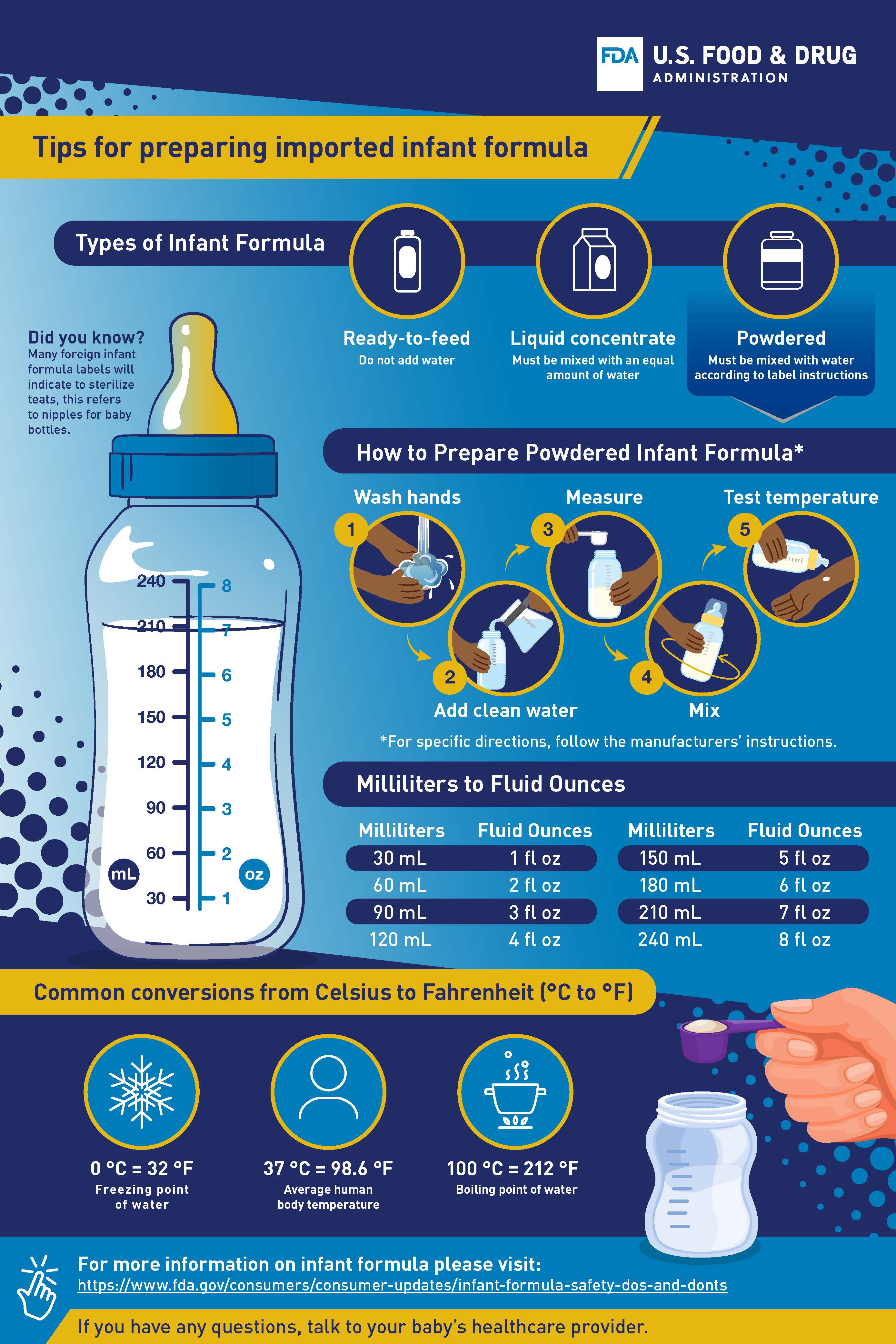
- Sharing tips with consumers on how to safely prepare imported infant formula.
Tips for Preparing Imported Infant Formula
Consumer Information
- List of formulas now allowed in the U.S. under FDA enforcement discretion
- What are Counterfeit Infant Formulas?
- Infant Formula Guidance Documents & Regulatory Information
- Once Baby Arrives from Food Safety for Moms to Be
- Infant Formula: Safety Do's and Don'ts
- FDA Advises Parents and Caregivers to Not Make or Feed Homemade Infant Formula to Infants
- May 25, 2022 - Congressional Testimony on Formula Safety and Supply: Protecting the Health of America's Babies