COMPANY ANNOUNCEMENT
Bayer Issues Voluntary Recall of Alka-Seltzer Plus® Products
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionMislabeling-The ingredients on the front sticker may not match the actual product in the carton
- Company Name:
- Bayer
- Brand Name:
-
Brand Name(s)Bayer
- Product Description:
-
Product DescriptionAlka-Seltzer Plus
Company Announcement
Bayer is voluntarily recalling Alka-Seltzer Plus® packages that:
- Were sold only in the U.S. at Walmart, CVS, Walgreens and Kroger (including Dillons Food Stores, Fred Meyer, Fry’s Food Stores, Ralphs, King Soopers and Smith’s Food and Drug) after February 9, 2018.
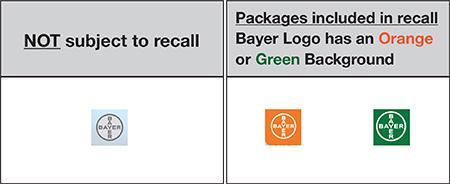
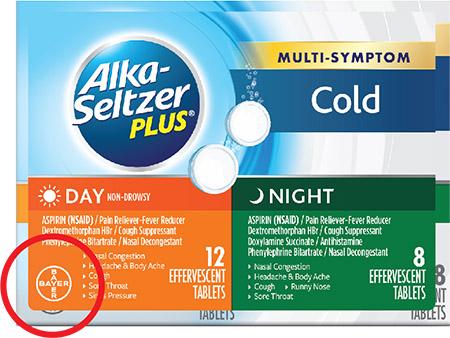
- Can be identified by checking the Bayer logo located on the lower left corner of the front of the carton. If the logo has an orange or green background, the product is included in the recall (please see attached photos).
The affected packages are being recalled because the ingredients on the front sticker may not match the actual product in the carton.
The ingredients listed on the front sticker of the carton may potentially be different from the ingredients listed on the back of the carton. This may lead consumers to ingest a product to which they may have an allergy or anaphylactic reaction, an ingredient which may be contraindicated for their medical condition or they intend to otherwise avoid. There may be potential for serious health consequences. To date, no complaint has been received that resulted in an adverse health consequence.
The Alka-Seltzer Plus products subject to the recall are intended to temporarily relieve symptoms associated with cold and flu, such as cough, congestion, fever and/or mucus.
Bayer is notifying retailers electronically and by certified mail and is arranging for return of all recalled product.
Consumers who purchased packages of Alka-Seltzer Plus that are being recalled should stop using the product and contact Bayer with questions, to report any issues experienced or for instructions about how to receive a refund.
Consumers with questions about this recall can contact Bayer Consumer Relations at: 1-800-986-0369 (available Monday - Friday 9:00 AM - 5:00 PM ET). Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
How To Identify Alka-Seltzer Plus Packages Subject To This Recall
View the front panel of any Alka-Seltzer Plus product purchased after Feb 9, 2018, and locate the Bayer Logo on the lower left corner.
If the Logo has an Orange or Green background, IT IS INCLUDED in this Recall.
Company Contact Information
- Consumers:
- Bayer Consumer Relations
- 1-800-986-0369
- Media:
- Jennifer Brendel
- 607-859-2227
- jennifer.brendel@bayer.com