Center for Drug Evaluation and Research Response to Coronavirus (COVID-19) | Infographic
Visualization of data associated with CDER's response to the coronavirus (COVID-19) pandemic from January 1, 2020 – February 1, 2021
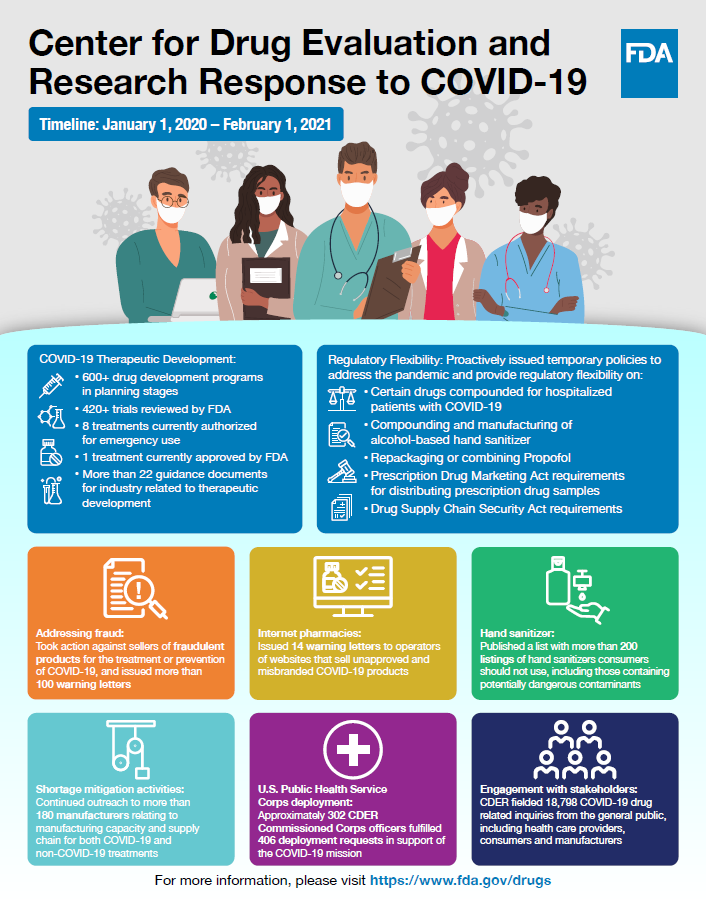
COVID-19 Therapeutic Development
- 600+ drug development programs in planning stages
- 420+ trials reviewed by FDA
- 8 treatments currently authorized for emergency use
- 1 treatment currently approved by FDA
- More than 22 guidance documents for industry related to therapeutic development
Regulatory Flexibility
Proactively issued temporary policies to address the pandemic and provide regulatory flexibility on:
- Certain drugs compounded for hospitalized patients with COVID-19
- Compounding and manufacturing of alcohol-based hand sanitizer
- Repackaging or combining Propofol
- Prescription Drug Marketing Act requirements for distributing prescription drug samples
- Drug Supply Chain Security Act requirements
Addressing fraud
Took action against sellers of fraudulent products for the treatment or prevention of COVID-19 and issued more than 100 warning letters
Internet pharmacies
Issued 14 warning letters to operators of websites that sell unapproved and misbranded COVID-19 products
Hand sanitizer
Published a list with more than 200 listings of hand sanitizers consumers should not use, including those containing potentially dangerous contaminants
Shortage mitigation activities
Continued outreach to more than 180 manufacturers relating to manufacturing capacity and supply chain for both COVID-19 and non-COVID-19 treatments
U.S. Public Health Service Corps deployment
Approximately 302 CDER Commissioned Corps officers fulfilled 406 deployment requests in support of the COVID-19 mission
Engagement with stakeholders
CDER fielded 18,798 COVID-19 drug related inquiries from the general public, including health care providers, consumers and manufacturers
For more information, please visit www.fda.gov/drugs