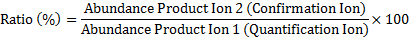BAM: Quantitative Analysis for Cereulide in Food Products
<< BAM Chapter 14: Bacillus cereus
Authors: Sandra M. Tallent CFSAN/ORS/DM/MMSB and Ann Knolhoff CFSAN/ORS/DAC/SMSB
Bacillus cereus is a Gram-positive spore-forming rod naturally found in soil. The ubiquitous and resistant spores permit contamination of many food products and are a major concern in food spoilage prevention. The bacteria and spores are often detected in rice, pasta, dehydrated foods, meats, vegetables and dairy products. Dairy products are especially susceptible to spoilage because spores can survive the pasteurization process (Schoeni and Wong 2005).
Intoxication is caused by the pre-formed cereulide produced by B. cereus spores after germinating in food that has not been stored properly. Ingestion of the pre-formed toxin typically begets nausea and vomiting. The cereulide is resistant to acid, heat and digestive enzymes. The cyclic dodecadepsipeptide acts as an ionophore disrupting oxidative phosphorylation in the mitochondria and has been associated with several cases of liver failure (Mahler et al. 1997; Dierick et al. 2005; Pósfay-Barbe et al. 2008).
This method briefly outlines the cereulide method; please access the American National Standards Institute’s (ANSI) reference portal site, Incorporated by Reference (IBR), to acquire the full standard or review for ISO 18465:2017. FDA employees can also access the ANSI website through FDA library.
1. SCOPE
This method is applicable to all scientists that perform the method for the detection and quantification of cereulide. The cereulide is extracted from the food matrix with acetonitrile. Labeled cereulide is used as the internal standard. Cereulide is detected with UHPLC/ESI-MS/MS in positive mode. The food matrices subjected to validation included: cooked rice both spiked and naturally contaminated, fried rice, cream pastry with chocolate, hotdog sausage, mini pancakes, vanilla custard and infant formula.
2. DEFINIONS/ACRONYMS/GLOSSARY
Cereulide a pre-formed toxin produced by some strains of Bacillus cereus, which is a cyclic dodecadepsipeptide.
- LC liquid chromatography
- MS mass spectrometry
- MS/MS tandem mass spectrometry
- MRM multiple reaction monitoring
- ESI electrospray ionization
- IS internal standard
3. RESPONSIBILITIES
SOP Responsible Official: Sandra Tallent and Ann Knolhoff
4. MATERIALS
- Chemicals and reagents shall be LC-MS grade and/or 98-100% pure.
- Water
- Acetonitrile
- Methanol
- Formic acid
- Ammonium formate
- Synthetic cereulide standard and and its labelled isotope 13C6-Cereulide; both are commercially available (Chiralix B.V., Nijmegen, Netherlands; purity > 95% using 1H NMR/LC)
- Prepared solutions: refer to ISO 18465:2017 for full description.
- Mobile phase A: 10 mM ammonium formate with 0.1% formic acid
- Mobile phase B: acetonitrile with 0.1% formic acid
- Internal standard (IS) stock solutions with labeled cereulide 13C6 –cereulide. All standards should be stored at -20 oC.
- IS-A, 100,000 ng/mL: 10 mg of synthetic cereulide in 100 ml of methanol
- IS-B, 1000 ng/mL: dilute IS-A with methanol to prepare
- IS-C, 100 ng/mL: dilute IS-A with acetonitrile to prepare
- IS-D, 10 ng/mL; dilute IS-B with acetonitrile to prepare
- Cereulide standard stock solutions: prepare similarly to IS solutions
- Cer-A, 100,0000 ng/mL in methanol
- Cer-B, 100 ng/mL in acetonitrile
- Cer-C, 10 ng/mL in acetonitrile
- Standards for calibration curve. Refer to the table below and to ISO 18465:2017 for full description.
5. EQUIPMENT
Please access the American National Standards Institute’s (ANSI) reference portal site, Incorporated by Reference (IBR), to acquire the full standard or review https for ISO 18465:2017 for more specific details. FDA employees can also access the ANSI website through FDA library.
- MS system with multiple reaction monitoring (MRM) capability and compatible ESI interface, such as ABSciex 5500 or equivalent
- LC, such as Waters Acquity or equivalent
- C18 Column, such as Phenomenex Kinetex C18, 100 x 2.10 mm, 1.7 µm, 100 Å, Supelco Discovery C18, 100 mm x 2.1 mm, 5 µm, or equivalent
- Centrifuge
- Polypropylene conical tubes (50 mL)
- Horizontal shaking device
- Analytical scale (accuracy 0.00001 g)
- Blender
- Adjustable volume pipettes (needed ranges are found in Table 1)
- Pipets with pipet tips
- LC autosampler vials
- PTFE membrane filters, 0.20 µm
- Vortex mixer
- Centrifuge tubes (2 mL)
- Centrifuge
- Volumetric glassware to prepare solutions listed under Section 5.
6. METHOD
Please access the American National Standards Institute’s (ANSI) reference portal site, Incorporated by Reference (IBR), to acquire the full standard or review for ISO 18465:2017 for more specific details. FDA employees can also access the ANSI website through FDA library.
- Sample preparation
Sample should be stored at -20oC. Transfer 100 g of sample to a sample container for testing and homogenize as described Ch 1 BAM.
Weigh 2.500 + 0.005 g of the homogenized sample to a 50 mL conical centrifuge tube and pipette 500 µL internal standard solution IS-C to the weighed sample. Prepare duplicate samples for each sample tested. Vortex the samples on high speed for 10 seconds and allow tubes to equilibrate and settle for 30 minutes at room temperature. Pipet 29.5 mL of acetonitrile to each sample, tighten the lid, shake each sample for 30 seconds by hand before placing on a horizontal shaking device. Shake vigorously for 1 hour. Adjust the volume to maintain the same ratio for smaller sample volumes.
Centrifuge the samples at room temperature for 10 minutes at 1000-1500 x g. Filter 2 mL of each sample through a PTFE membrane filter and transfer to a glass autosampler vial and cap with a lid. Alternatively, 2 mL of sample can be centrifuged at 10,000 g to 12,0000 g for 10 min. Prepare the instrument as required by the manufacturer.
- LC Conditions
An isocratic method with a flow rate of 0.3 mL/min with 10/90 mobile phase A/B can be used with a column temperature of 40oC and injection volume of 5 µL.
- MS
Mass Spectrometry settings can vary between instruments. The following are example settings for an ABSciex 5500 instrument: entrance potential10 V; curtain gas 20; Collision energy 80 V for m/z 499 and 90 V for m/z 314; Collision gas flow medium; IonSpray voltage 5.5 kV; Desolvation temperature 700oC; Desolvation gas flow 40 (ion source gas 1) and 70 (ion source gas 2).
The precursor ion for cereulide is m/z 1170.7 and the transitions are m/z 314.4 for the quantitative product ion and m/z 499.4 for the confirmation ion. The precursor ion for 13C6 –cereulide is m/z 1176.7 and the quantitative product ion is m/z 172.2.
- Calculations and Reporting
A calibration line is made from the cereulide standards and the line is forced through the origin. The calculation method is set as internal standard method. The calibration line allows quantification of the samples which fall within the range of the calibration line. If a sample is out of the range, less sample weight has to be applied. The concentration (X, ng/mL) is calculated using the data processing software using a response factor to compensate for the measured amount of internal standard.
The cereulide level in the sample can be calculated with the formula of the calibration line using the equation below:
The ion ratio in standards and samples can be calculated using the equation below:
LOD (Limit of Detection): The calculated concentration in the solution (or the concentration recalculated to the original sample) in the case that the signal of product ion 2 is 3x the background noise.
LOQ (Limit of Quantification): The calculated concentration in the solution (or the concentration recalculated to the original sample) in the case that the signal of product ion 2 is 6x the background noise.
- Quality Checks
Check if both product ions from cereulide in a sample exist and meet the LOD - LOQ requirements; if not, there is no positive identification or measurement possible.
Check if the ion ratio from the cereulide product ions (Rs) in a sample meet the criteria for conformation of identity (established in EU-regulation 2002/657/EG European commission). For a positive identification the ratio of product ions in a sample has to lie within a range, which is established by the average ratio of the product ions in the standards / reference (Rr) and a required tolerance interval. This interval is dependent on the level of the average ratio in the standards / references.
Ratio (Rr) in Reference > 50 %: Positive identification, ratio sample (Rs) lies within Rr ± 20%.
Ratio (Rr) in Reference > 20 to 50 %: Positive identification, ratio sample (Rs) lies within Rr ± 25%.
Ratio (Rr) in Reference > 10 to 20 %: Positive identification, ratio sample (Rs) lies within Rr ± 30%.
Ratio (Rr) in Reference < 10 %: Positive identification, ratio sample (Rs) lies within Rr ± 50%.
The change in retention times during a set of measurements may not exceed the average retention time of the measurements of the standard ± 2.5%.
Investigate for every series of samples the positive sample control and check if the results corresponds with the settled criteria (Shewart control chart ).
Check if the area of the peak of product ion 2 of the lowest concentration standard is at least 6 times the background noise (≥ LOQ) (Signal to noise ratio ≥ 6).
Check if the correlation coefficient ( r ) of the linear calibration line is at least 0.995.
The standard with a concentration of 8.25 ng/ml is measured every 10 samples to check the trend in the signal of the instrument (declining peak area). The decline of the peak area should be less than 10 %.
As one of the above checks does not meet the quality requirements, inspect your method.
7. FIGURES AND TABLES
- Standard preparation for standard curve. Transfer volumes of the stock solution Cer-C, acetonitrile, and labeled cereulide 13C6-cereulide IS-D into a vial and cap. Vortex the standard for 20 seconds. Standard concentrations should be modified to reflect the actual weighed amount of cereulide used in Cer-A and subsequent standard solutions.
µL Cer-C µL acetonitrile µL IS-D Standard concentration 0 1000 200 0.000 ng/mL 10 990 200 0.08 ng/mL 50 950 200 0.4 ng/mL 100 900 200 0.8 ng/mL 200 800 200 1.7 ng/mL 500 500 200 4.2 ng/mL 1000 0 200 8.3 ng/mL
8. FORMS AND ATTACHMENTS
9. SUPPORTING DOCUMENTS
- ISO 18465:2017, Microbiology of the food chain – Quantitative determination of emetic toxin (cereulide) using LC-MS/MS.
10. REFERENCE DOCUMENTS
- Bauer, T., Stark. T., Hofmann, T., and Ehling-Schulz, M. (2010) Development of a stable isotope dilution analysis (SIDA) for the quantification of the Bacillus cereus toxin cereulide in foods. Agric. Food Chem. 58: 1420-1428.
- Biesta-Peters, E.G., Reij, M.W., Blaauh, R.H., in ‘t Veld, P.H., Rajkovic, A., Ehling-Schuulz, M. and Abee, T. (2006). Quantification of the emetic toxin cereulide in food products by liquid-chromatography mass spectrometry using synthetic cereulide as a standard. Appl and Env. Microbiol. 76:22, 7466-7472.
- Dierick, K. Van Coillie, E., Swiecicka, I., Meyfroidt, G., Devlieger, H., Meulemans, A., Hoedemaekers, G., Fopurie, L., Heyndricks, M., and Mahillon, J. (2005). Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 43: 4277–4279.
- Häggblom, M.M., Apetroiae, C., Andersson M.A., and Salkinoja-Salonen, M.S. (2002). Quantitative Analysis of Cereulide, the Emetic Toxin of Bacillus cereus, Produced under Various Conditions. Appl. And Env. Microbiol. 68: 2479-2483.
- Jääskeläinen, E.L., Häggblom, M.M., Andersoon, M.A., Vanne, L., and Salkinoja-Salonen, M.S. (2003). Potential of Bacillus cereus for producing an Emetic Toxin, Cereulide, in Bakery Products: Quantitative Analysis by Chemical and Biological Methods. J. Food Prot. 66: 1047-1054.
- Mahler, H., Pasi, A., Kramer, J.M., Schulte, Pl, Scoging, A.C., Bar, W. and Krahenbuhl, L.S. (1997). (1997). Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N. Engl. J. Med. 336: 1142–1148.
- Posfay-Barbe, K.M., Schrenzel, J., Frey, J., Studer, R., Korff, C., Belli, D.C., Parvex, Pl, Rimensberger, P.C., and Schappi, M.G. (2008). Food poisoning as a cause of acute liver failure. Pediatr. Infect. Dis. J. 27: 846–847.
- Schoeni, J.L. and Wong, A.C. (2005). Bacillus cereus food poisoning and its toxins. J. Food Prot. 68: 636–648.
- Tallent, S.M., Hait, J.M., Knolhoff, A.M. Bennett, R.W., Hammack, T.S., and Croley, T. R. 2016. Rapid testing of food matrices for Bacillus cereus enterotoxins. J. Food Safety. n/a, e12292. doi:10.1111/jfs.12292.