AURA3: Taste changes
Project Patient Voice is intended to be used with a healthcare professional when discussing the potential symptoms related to a cancer and cancer treatment. Do not rely on Project Patient Voice alone to make decisions about medical care. Do not use Project Patient Voice to substitute for advice from your health care professional. Conclusions about patient experiences with symptoms may be limited because not all symptoms may have been captured by the patient-reported questionnaire.
Download symptom data (XLSX, 24KB)
In AURA3 Study, Patients Were Asked: "In the last 7 days, what was the SEVERITY of your PROBLEMS WITH TASTING FOOD OR DRINK at its WORST?"
Patients scored the severity of their Taste Changes on a 5-point scale (None, Mild, Moderate, Severe, Very Severe)
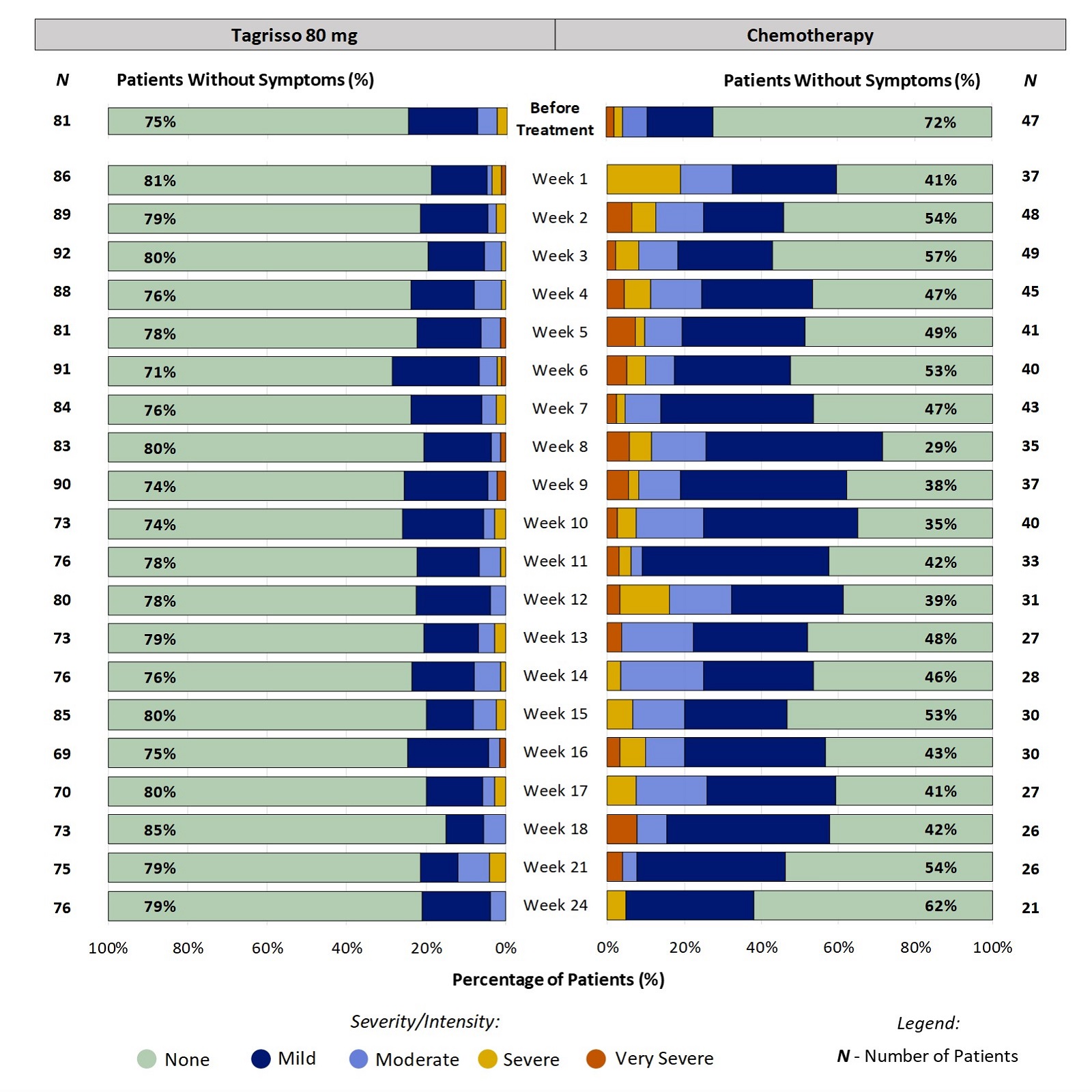
Patient-Reported Taste Changes During the First 24 Weeks on Treatment for Patients Who Completed a Questionnaire:
Figure 1 shows the percentage of patients reporting the severity of their Taste Changes at each time point. For example, at week 2, 21% of patients taking Tagrisso reported Taste Changes (ranging from Mild to Severe). The range of patients who had any Taste Changes during the first 24 weeks of treatment with Tagrisso was between 15% - 29%. Click here for more information on how to read the graphs below.
Figure 1. Patient-Reported Taste Changes During the First 24 Weeks on Treatment
All responses from patients' experiences just before and up to week 24 on-treatment were included in the analysis. Some patients did not report their symptoms every week, therefore the number of patients may vary between weeks. Furthermore, not all patients remained on the treatment for 24 weeks (e.g., some stop treatment for worsening disease) which is a reason for the change in the number of patients over the course of treatment.
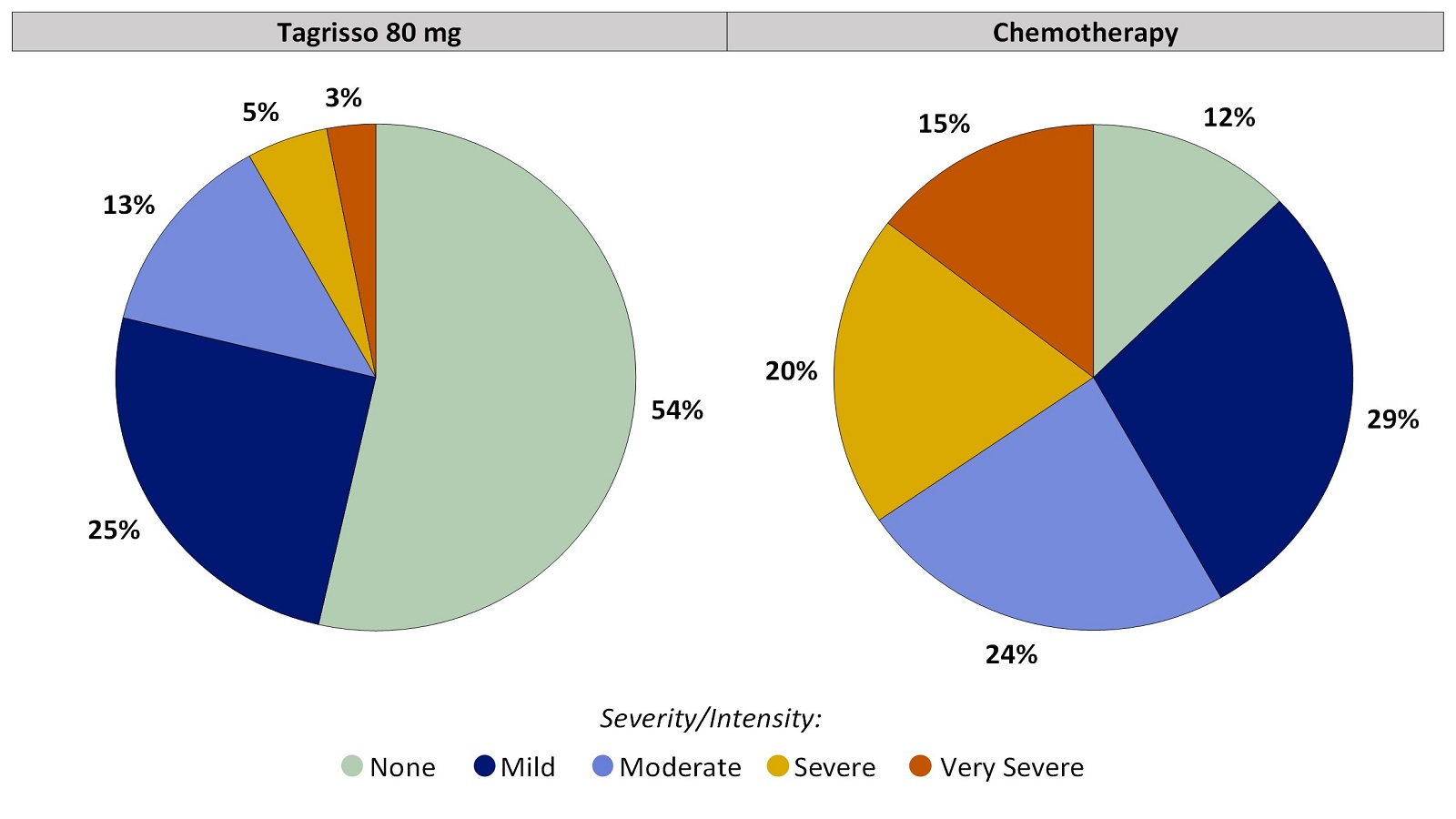
Worst Response Option for Taste Changes That Patients Reported During the First 24 Weeks on Treatment
Figure 2. Worst Patient-Reported Taste Changes During the First 24 Weeks on Treatment
Patients with at least one on-treatment Taste Changes score were included in the analysis. Tagrisso (N=99), Chemotherapy (N=55).
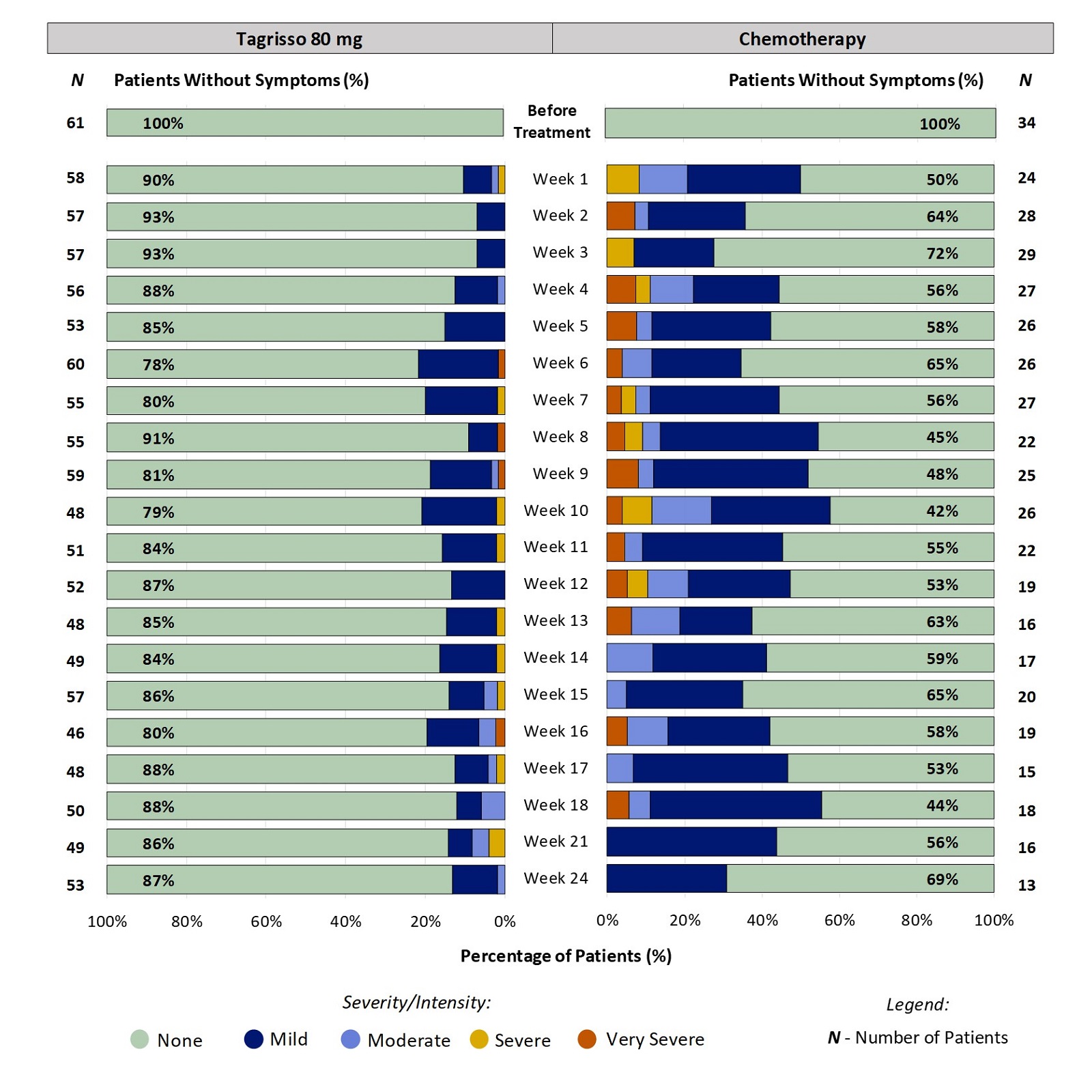
Some Patients Did Not Report Taste Changes Before Treatment:
For patients that did not report Taste Changes before treatment, Figure 3 shows the percentage of patients reporting the severity of their Taste Changes between weeks 1 and 24.
Figure 3. Patient-Reported Taste Changes During the First 24 Weeks on Treatment: Patients Without Taste Changes Before Treatment
All responses from patients who did not report Taste Changes before treatment were included in the analysis. Some patients did not report their symptoms every week, therefore the number of patients may vary between weeks. Furthermore, not all patients remained on the treatment for 24 weeks (e.g., some stop treatment for worsening disease) which is a reason for the change in the number of patients over the course of treatment.
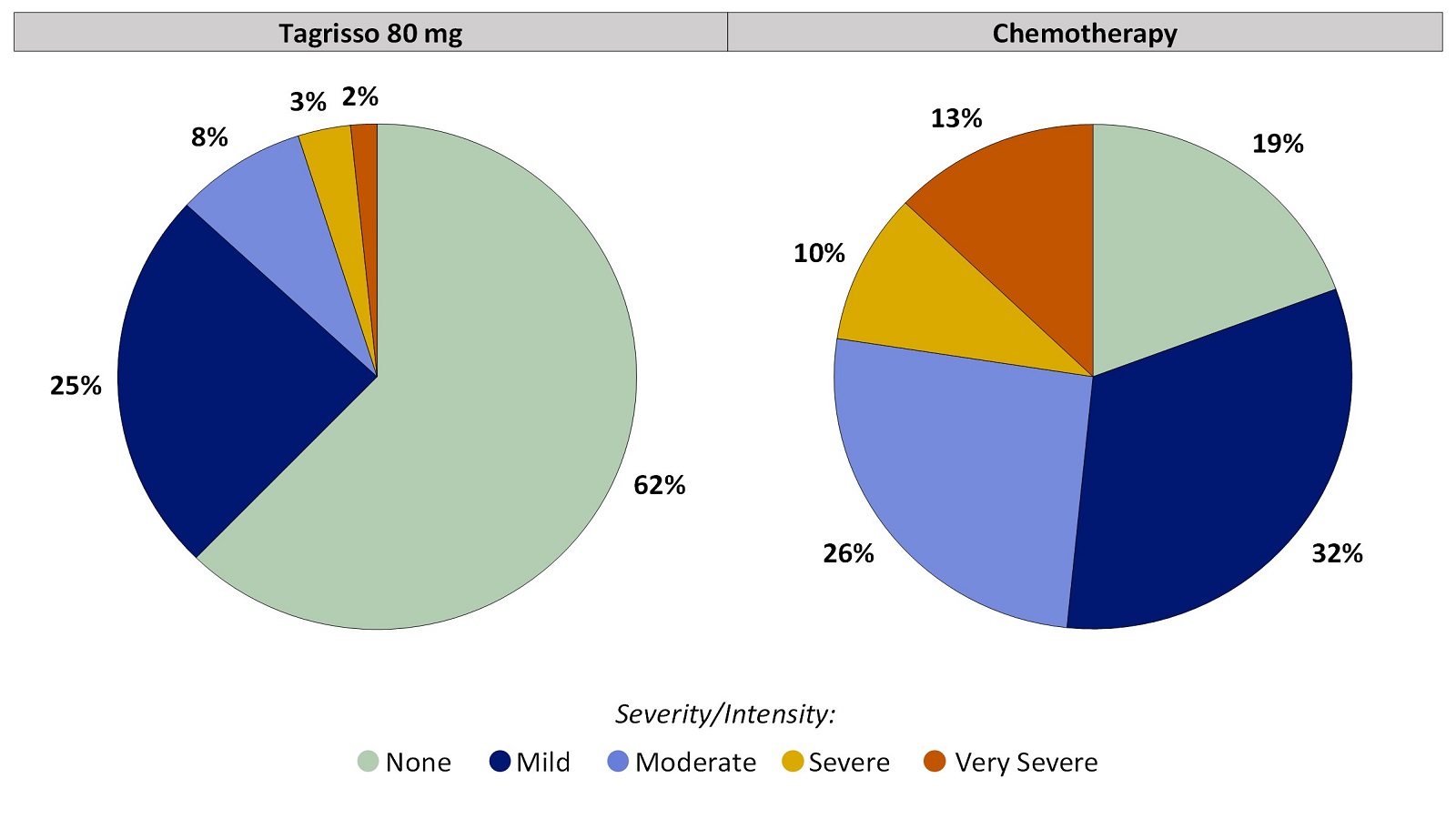
Worst Response Option for Taste Changes That Patients Reported During the First 24 Weeks on Treatment, for Patients Who Did Not Have Taste Changes Before Treatment:
Figure 4. Worst Patient-Reported Taste Changes During the First 24 Weeks on Treatment: Patients Without Taste Changes Before Treatment
Patients who had no Taste Changes before treatment and at least one on-treatment Taste Changes score were included in the analysis. Tagrisso (N=61), Chemotherapy (N=31).