Expanded Access (Compassionate Use) Submission Data Archive FY 14 - 18
CDER and CBER Archived Data (2014-2018)
CDRH Expanded Access Requests (2014-2018)
CDER and CBER Archived Data (2014-2018)
CBER and CDER Tables
FY 2014 – 2018 Graphs of Expanded Access CDER and CBER Submissions
To view the graph select the appropriate link below.
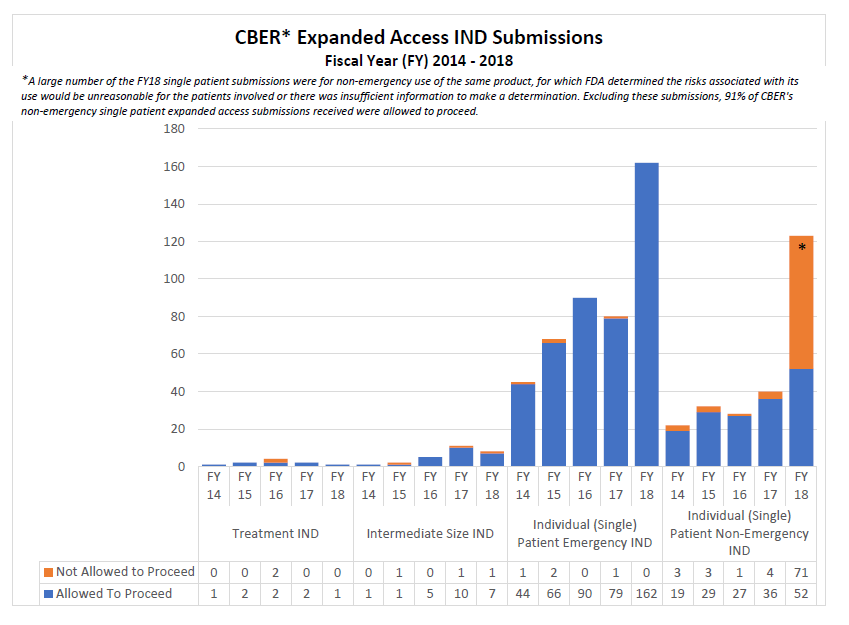
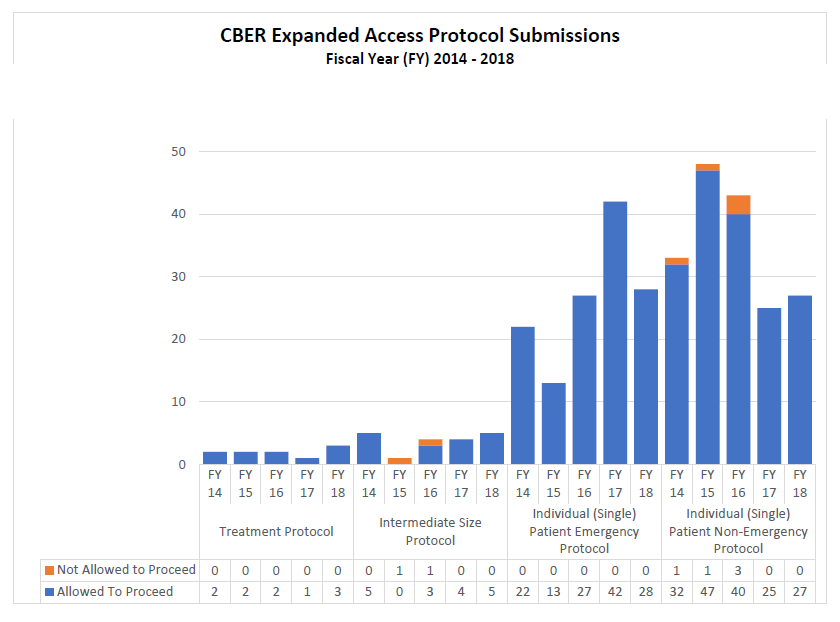
- 2014-2018 CBER Expanded Access INDs and Protocols (2014-2018)
- 2014-2018 CDER Expanded Access INDs and Protocol (2014-2018)
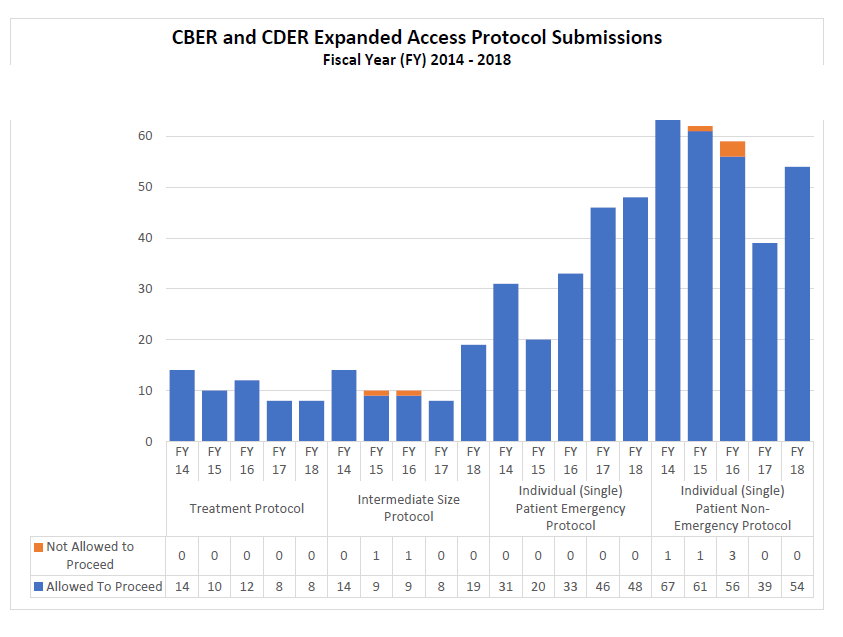
- 2014-2018 Combined CDER and CBER Expanded Access Submissions and Protocols (2014-2018)
CBER and CDER Tables
CBER and CDER Expanded Access INDs (2014-2018)
* A large number of the single patient expanded access submissions were for non-emergency use of the same product, for which FDA determined the risks associated with its use would be unreasonable for the patients involved or there was insufficient information to make a determination. Excluding these submissions, 91% of CBER’s non-emergency single patient expanded access submissions received were allowed to proceed.
For tables with older data, see archive page.
Expanded Access Protocols for CDER and CBER (2014-2018)
For tables with older data, see archive page.
FY 2014 – 2018 Graphs of Expanded Access CDER and CBER Submissions
2014-2018 CBER Expanded Access INDs and Protocols (2014-2018)
2014-2018 CDER Expanded Access INDs and Protocol (2014-2018)
2014-2018 Combined CDER and CBER Expanded Access Submissions and Protocols (2014-2018)
CDRH Expanded Access Requests (2014-2018)
FY 2014-2018 Graph of CDRH Expanded Access Requests
CDRH IDE Expanded Access Requests (2014-2018)1
| Expanded Access Totals | |||||
|---|---|---|---|---|---|
| FY 2014 | FY 2015 | FY 2016 | FY 2017 | FY 2018 | |
| Requested Received | 218 | 222 | 136 | 100 | 79 |
| Evaluable Requests2 | 212 | 211 | 124 | 96 | 69 |
| Approved | 211 | 210 | 123 | 96 | 69 |
| Percentage Approved3 | 99.5% | 99.5% | 99.2% | 100.0% | 99.6% |
1 Expanded Access IDE requests reflect compassionate use (single patient or small population) request supplements to existing IDEs. The Treatment IDE option is generally not used, and Emergency Use may proceed without prior FDA approval.
2 Evaluable requests are those sufficiently complete for FDA’s review and resulted in a decision to approve or deny.
3 Based on approved requests to total number of evaluable requests.
CDRH Non-IDE Expanded Access Requests(2014-2018)1
| Expanded Access Totals | |||||
|---|---|---|---|---|---|
| FY 2014 | FY 2015 | FY 2016 | FY 2017 | FY 2018 | |
| Requested Received | 179 | 167 | 198 | 230 | 257 |
| Evaluable Requests2 | 167 | 155 | 196 | 220 | 233 |
| Approved | 165 | 154 | 196 | 220 | 232 |
| Percentage Approved3 | 98.8% | 99.4% | 100.0% | 100.0% | 99.6% |
1 Expanded Access requests outside of an IDE are limited to single patient compassionate use requests.
2 Evaluable requests are those sufficiently complete for FDA’s review and resulted in a decision to approve or deny.
3 Based on approved requests to total number of evaluable requests.