FY2015 Regulatory Science Research Report: Complex Mixtures and Peptides
Introduction
One category of complex generics has complex active ingredients such as peptides, complex mixtures, or natural source products. For complex active ingredients, our research goal is to apply modern analytical and quantitative analysis methods to characterize product-specific attributes so that the sameness of the active ingredient can be established between the RLD and the proposed generic drug. For most peptide drugs, the active ingredient is clearly defined and can be well characterized. However, characterizing the impurity profile of peptide-related substances and assessing the associated safety risks, including immunogenicity of a proposed generic product, is challenging; this also is a goal of our current research.
Research
In 2014, ORS worked closely with the Office of Testing and Research (OTR) and the Office of Biotechnology Products (OBP) on a number of internal and external (supported by grant mechanisms) research projects. Collaborations with OTR characterized complex mixtures and peptides. These projects included analyzing complex mixtures such as conjugated estrogens, glatiramer acetates, and sevelamer. Based on the outcomes of these projects, the draft guidances on conjugated estrogens and sevelamer were published in 2014. These guidances provided the criteria for establishing sameness of an active ingredient and laid out a clear path for the development of a generic product.
Our research on glatiramer acetate resulted in methodologies that can quantitatively assess differences in glatiramer acetate-related products. These methods made significant contributions in the review and approval of the first generic glatiramer acetate product. For peptide-related impurity analysis, OTR developed a UPLC-HRMS-based method that has much higher resolution than the conventional HPLC-UV method and is suited for peptide drug quality control. In order to evaluate the immunogenicity risk of peptide drugs, ORS collaborated with OBP in a project to assess the innate immune response that modulates impurities in peptide drugs.
Additionally, ORS coordinated with the Office of Pharmaceutical Quality to support research projects characterizing complex drug products containing complex mixtures and developing mathematical models to assess critical quality attributes through external grants. FDA/OGD/ORS issued three grants in 2014 to study specific complex drug products, including pentosan polysulfate and crofelemer. Collectively, these research activities advance FDA’s understanding of complex drug products containing complex mixtures, help establish product-specific guidance on active ingredient sameness, and provide critical inputs to regulatory review and decision-making processes.
ORS staff facilitating research in this area
- Xiaohui Jiang, Meng Hu, Deyi Zhang, and other ORS staff members
Projects and Collaborators
- Integrated approach to determine equivalence in complex drug mixtures, Massachusetts Institute of Technology
- Site PI: Ram Saisekharan
- Grant #: 1U01FD005291
- Development of an integrated mathematical model for comparative characterization of complex molecules, University of Kansas Lawrence
- Site PI: David Volkin
- Grant #: 1U01FD0058285
- Statistical methodology for characterization of macromolecular similarity, Battelle Pacific Northwest Laboratories
- Site PI: John Cort
- Grant #: 1U01FD005288
- Internal Project: Characterization of glatiramer acetate
- FDA Collaborator: David Keire
- FDA Center/Office/Division: CDER/OPQ/OTR/DPA
- Internal Project: Characterization of impurities in synthetic peptides
- FDA Collaborator: Michael Boyne
- FDA Center/Office/Division: CDER/OPQ/OTR/DPA
- Internal Project: Characterization of sequence and impurity of H.P. Acthar gel
- FDA Collaborator: Ashley Gucinski
- FDA Center/Office/Division: CDER/OPQ/OTR/DPA
- Internal Project: Assessment of innate immune response modulating impurities in peptide drugs
- FDA Collaborator: Daniela Verthelyi
- FDA Center/Office/Division: CDER/OPQ/OBP
Publications and Presentations
- Kui Zeng, Ilan Geerlof-Vidavisky, Ashley Gucinski, Xiaohui Jiang and Michael T. BoyneII, Liquid chromatography-high resolution mass spectrometry for peptide drug quality control, AAPS J. 17(3), 643-51 (2015)
- Michaella J. Levy, Michael T. BoyneII, Sarah Rogstad, David J. Skanchy, Xiaohui Jiang and Ilan Geerlof-Vidavsky1, Marketplace analysis of conjugated estrogens: determining the consistently present steroidal content with LC-MS, AAPS J. DOI 10.1208/s12248-015-9805-x (2015)
- Sarah Rogstad, Eric Pang, Cynthia Sommers, Meng Hu, Xiaohui Jiang, David A. Keire and Michael T. Boyne II, Modern analytics for synthetically derived complex drug substances: NMR, AFFF–MALS, and MS tests for glatiramer acetate, Analytical and Bioanalytical Chemistry. DOI 10.1007/s00216-015-9057-8 (2015)
Outcomes
- Draft Guidance on Conjugated Estrogens (Tablet). Recommended Dec 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM428204.pdf
- Draft Guidance on Sevelamer Carbonate (Suspension). Recommended Dec 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM261528.pdf
- Draft Guidance on Sevelamer Hydrochloride (Tablet). Recommended Dec 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm089621.pdf
- Approval of the first generic of glatiramer acetate injection on Apr 16, 2015 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm443143.htm
Figure 5. LC-MS chromatographic alignment of the resulting peptides from enzymatic digestion of glatiramer acetate samples from three Teva lots and a glatiramoid sample from Sigma (copolymer-1)

Five replicate injections of the Teva samples (blue – P53462, red – P53506, green – P53584) and copolymer-1 (yellow) were shown: (A) Alignment of the full LC-MS chromatography of all samples, demonstrating reproducible chromatography; and (B) at early elution times (from 0 to 10 minutes), there are distinct differences between the Teva lots and copolymer-1.
Figure 6. 3-D plot of all data points (three Teva lots and copolymer-1) in the constructed space by the first three principal components (which retain major variability/information of original data) after analyzing the LC-MS data in the figure above using a Sparse-PCA method
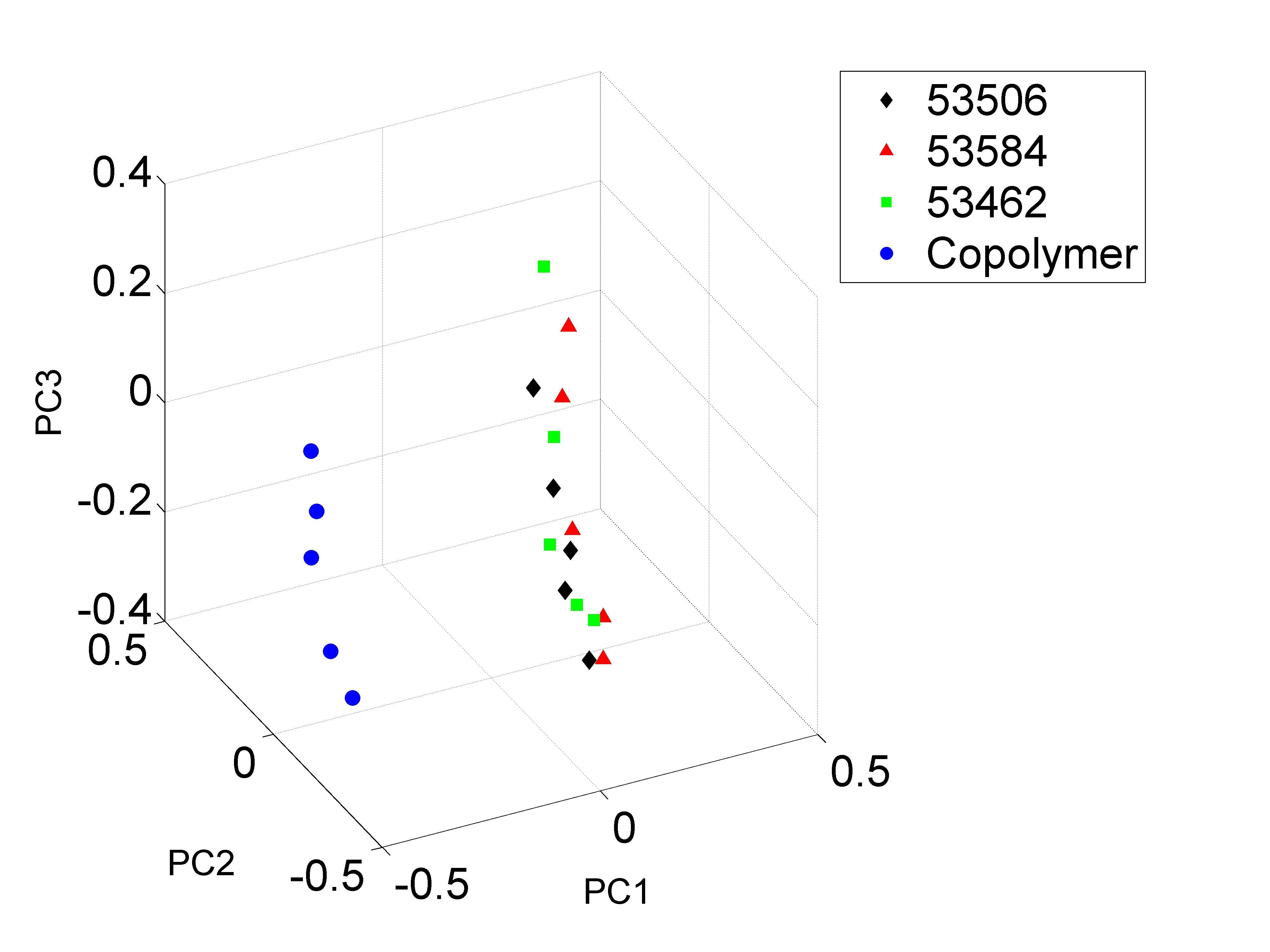
A clear separation between the Teva samples and copolymer-1 was observed.
