Vet-LIRN Cooperative Agreements
Currently Funded Opportunities
Vet-LIRN Network Infrastructure Grant Program (U18)
Infrastructure funding is key to making sure that the network can function and that laboratories can work to support Vet-LIRN’s mission. These awards facilitate participation in Vet-LIRN activities such as consumer complaint response, emergency exercises, proficiency tests, and laboratory accreditation. The agreements also increase the agency’s capability to analyze an increased number of samples in the event of animal food- or drug-related illnesses or other large-scale emergency events that require increased testing of implicated diagnostic or animal food samples. Cooperative agreements allow network laboratories to request additional funds if they are participating in a specific Vet-LIRN project, such as the Antimicrobial Resistance (AMR) monitoring program or if they are conducting whole-genome sequencing (WGS) work, or if their caseload is particularly heavy.
Vet-LIRN Network Capacity-Building Projects (U18)
The cooperative agreement is intended to support projects or equipment grants to help investigate potential issues with animal foods, as well as support work related to antimicrobial resistance and antimicrobial stewardship. Vet-LIRN Laboratories may apply for funding for research related to emerging public food safety issues identified by the Vet-LIRN and for equipment and personnel necessary to expand laboratory capability and capacity. This work may include the development and validation of new methods or work to support efforts related to antimicrobial resistance or antimicrobial stewardship. In 2021, a Notice of Special Interest was added to this announcement to request applications related to COVID-19 animal diagnostic work.
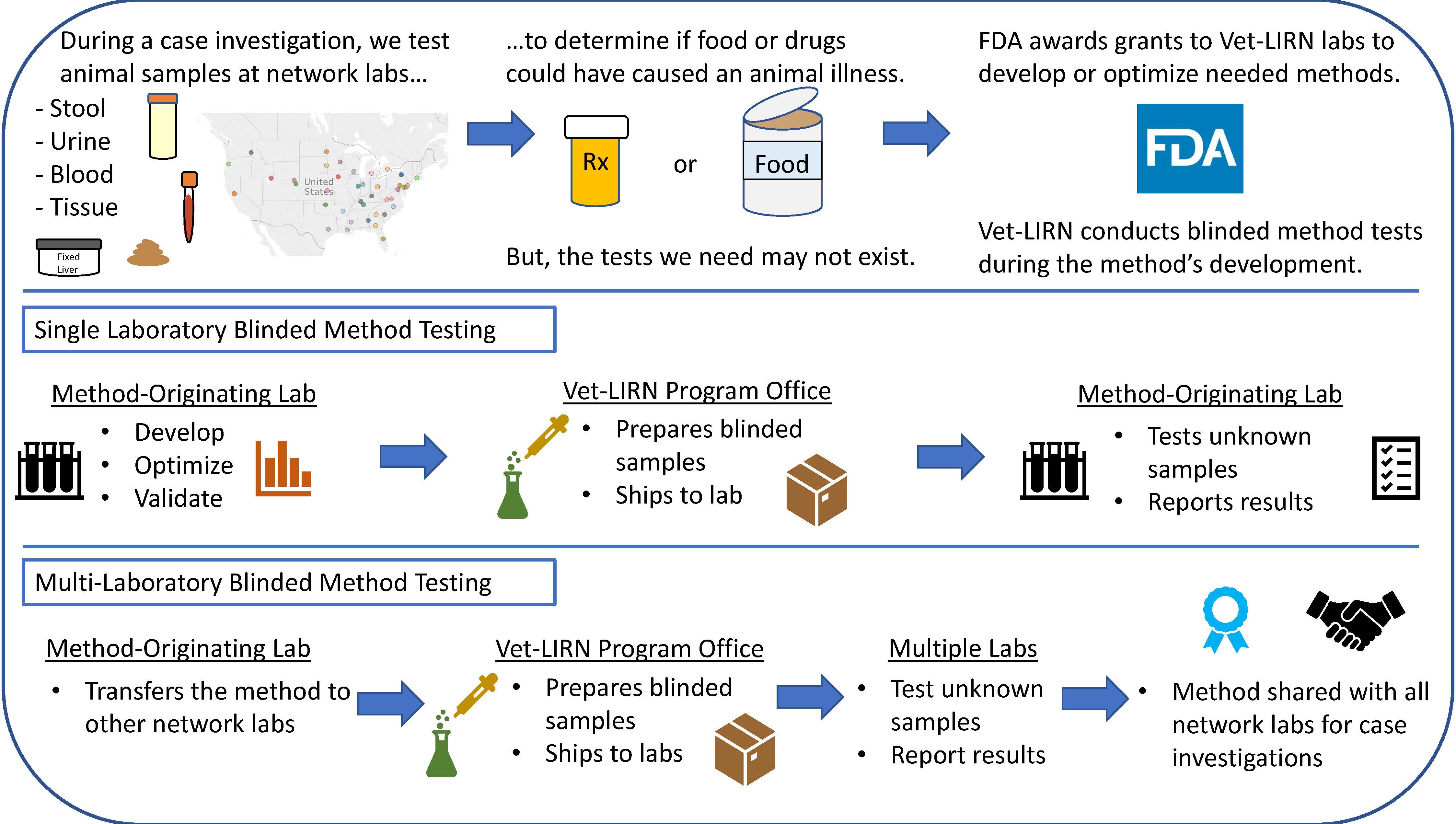
Figure 1. What are the steps during a Blinded Method Test?
Blinded Method Test
Testing animal diagnostic specimens enables Vet-LIRN to fully address consumer complaints about animal food or drugs. The Capacity Building grants and Blinded Method Tests ensure that the testing methods used in network laboratories are well evaluated and provide reliable data. Although Vet-LIRN methods are not regulatory methods, the FDA Method Validation Guidelines are generally followed during the method development and evaluation. Most of the projects have the following phases:
- Method-Originating (MO) Laboratory – Recipient of Cooperative Agreement Grant
- Method development/optimization.
- In-house validation using non-blinded samples.
- Vet-LIRN
- Vet-LIRN evaluates the method performance in the MO laboratory through exercises called Blinded Method Tests (BMTs). Vet-LIRN prepares and ships samples to the MO laboratory for blinded (i.e. unbiased) analysis. The MO laboratory analyzes blinded samples and submits results to organizers for evaluation of performance.
- MO Laboratory
- Method is transferred to other Vet-LIRN collaborating laboratories.
- Vet-LIRN
- Vet-LIRN evaluates the method performance in multiple laboratories in a Blinded Method Test.
The methods developed by our Vet-LIRN laboratories are extensively evaluated and will be
- shared with others via the data depository portal (www.protocols.io), published in a scientific journal, and
- adopted and used by Vet-LIRN laboratories for their routine animal diagnostic purposes and CVM consumer complaints.
Highlighted methods that completed BMTs 2020-2023 include
- North Dakota State University – Detection of SARS-CoV-2 in nasal swabs and lung homogenates from cats and dogs. Single-laboratory BMT was completed to evaluate the assay performance in the originating laboratory.
- University of California Davis - Quantitative determination of “Compound 1080” pesticide in bovine kidney using LC-MS/MS. Compound 1080 (sodium monofluoroacetate) is acutely toxic compound used for rodent and predator control. It is both man-made and is found naturally occurring in some tropical plants. Accidental poisoning of non-target animals does occur, and it is important to develop diagnostic tests to confirm exposure. The method was successfully evaluated during the single-laboratory BMT in the originating laboratory.
- University of Pennsylvania – Quantitation of aflatoxin B1 in fish food using lateral flow assay, which is based on the CHARM system. Mold can produce aflatoxin B1 in food ingredients such as grains (e.g. corn, wheat, barley), rice, nuts, and fruits. Aflatoxin B1 is the most toxic aflatoxin and is known for its mutagenic, carcinogenic, teratogenic, and immunosuppressive impact. It can cause death or intoxication in fish resulting in reduced growth rate and immunosuppression. Therefore, fast methods to identify this toxicant in fish food are desirable. The method was evaluated in both single-laboratory and multi-laboratory BMTs. In both BMTs samples were prepared by Vet-LIRN and analyzed in four collaborating laboratories.
- Kansas State University – Quantitation of the irradiation marker 2-DCB in dried chicken using GC-MS. Some manufacturers irradiate pet foods to kill bacterial and viral pathogens and extend food shelf life. However, improper food irradiation can result in microbial contamination of food products. Thus, having an effective method for determining irradiation dose is important to determine whether foods are properly irradiated.
- University of Pennsylvania – Detection of Clostridioides difficile in dog feces. Collaborator: PA laboratory
- Purdue University – Determination of three organophosphate pesticides (terbufos, diazinon and parathion) in bovine blood using GC-MS. Collaborator: IN laboratory