Drug Trials Snapshot: DAXXIFY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the DAXXIFY Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
DAXXIFY (daxibotulinumtoxin A)
dax' i fye
Revance Therapeutics, Inc.
Original Approval date: September 7, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DAXXIFY is a drug used in adults to temporarily improve the appearance of moderate to severe glabellar lines (wrinkles between the eyebrows).
How is this drug used?
DAXXIFY is given by a healthcare provider directly into the muscle through a needle (known as an intramuscular injection). It is injected into five different sites of the muscles of the frown lines. DAXXIFY should not be given more frequently than every three months.
Who participated in the clinical trials?
The FDA approved DAXXIFY based on evidence from two clinical trials (Studies GL-1 and GL-2), of 609 adult patients with moderate to severe glabellar lines (wrinkles between the eyebrows). The trials were conducted at 30 sites in the United States and Canada.
Who participated in the trials?
Table 1. Demographics (Studies GL-1 and GL-2)
|
Demographic |
Study GL-1 |
Study GL-2 |
Combined |
||
|---|---|---|---|---|---|
|
DAXXIFY |
Placebo |
DAXXIFY |
Placebo |
||
|
Age (years) |
|||||
|
Mean |
50.9 |
49.0 |
49.5 |
50.7 |
50.1 |
|
Range (min, max) |
23, 74 |
22, 74 |
21, 73 |
27, 75 |
21, 75 |
|
Age group, years, n (%) |
|||||
|
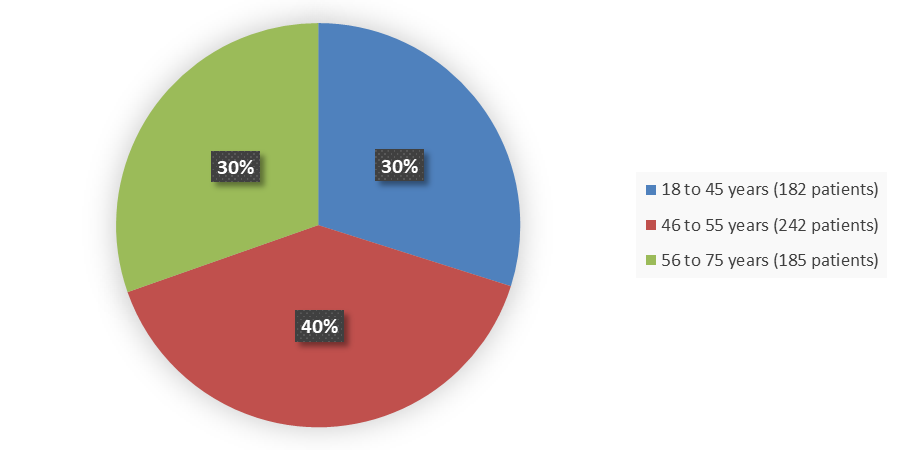
18 to 45 |
58 (29) |
32 (31) |
63 (31) |
29 (29) |
182 (30) |
|
46 to 55 |
68 (34) |
41 (40) |
91 (44) |
42 (42) |
242(40) |
|
56 to 75 |
75 (37) |
29 (28) |
51 (25) |
30 (30) |
185 (30) |
|
Sex, n (%) |
|||||
|
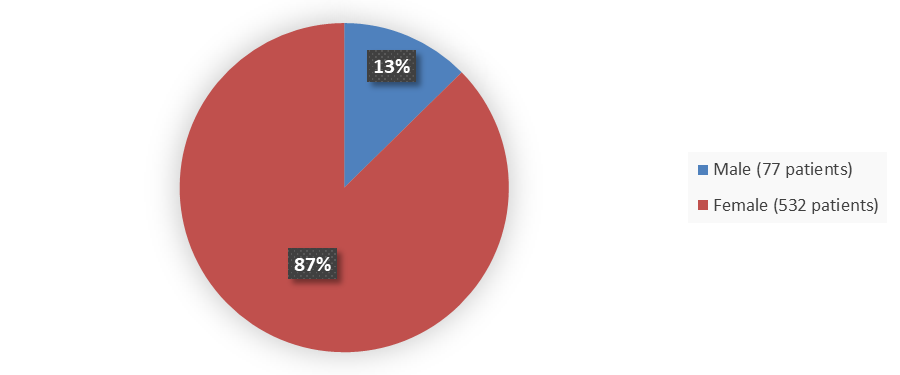
Female |
174 (87) |
88 (86) |
184 (90) |
86 (85) |
532 (87) |
|
Male |
27 (13) |
14 (14) |
21 (10) |
15 (15) |
77 (13) |
|
Race, n (%) |
|||||
|
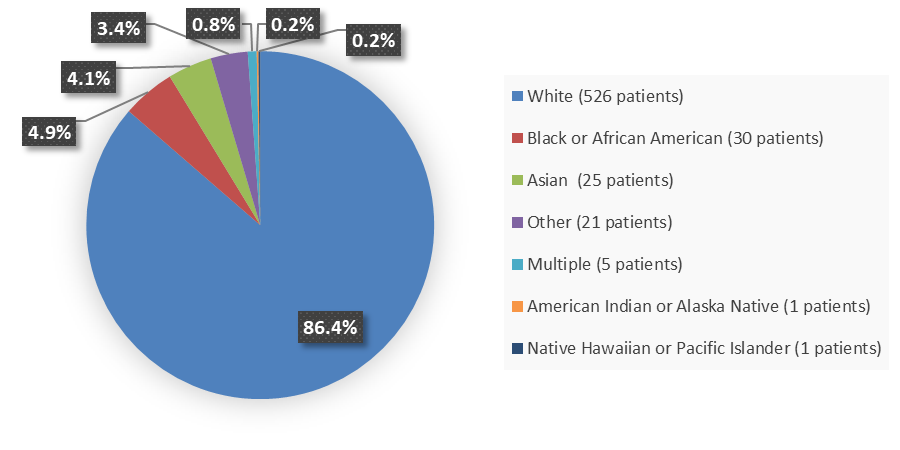
White |
173 (86) |
81 (79) |
181 (88) |
91 (90) |
526 (86) |
|
Black or African American |
10 (5) |
8 (8) |
9 (4) |
3 (3) |
30 (5) |
|
Asian |
7 (3) |
2 (2) |
11 (5) |
5 (5) |
25 (4) |
|
American Indian or Alaska Native |
0 |
1 (1) |
0 |
0 |
1 (<1) |
|
Native Hawaiian or Pacific Islander |
0 |
1 (1) |
0 |
0 |
1 (<1) |
|
Multiple |
1 (<1) |
2 (2) |
1 (<1) |
1 (1) |
5 (1) |
|
Other |
10 (5) |
7 (7) |
3 (11) |
1 (1) |
21 (4) |
|
Ethnicity, n (%) |
|||||
|
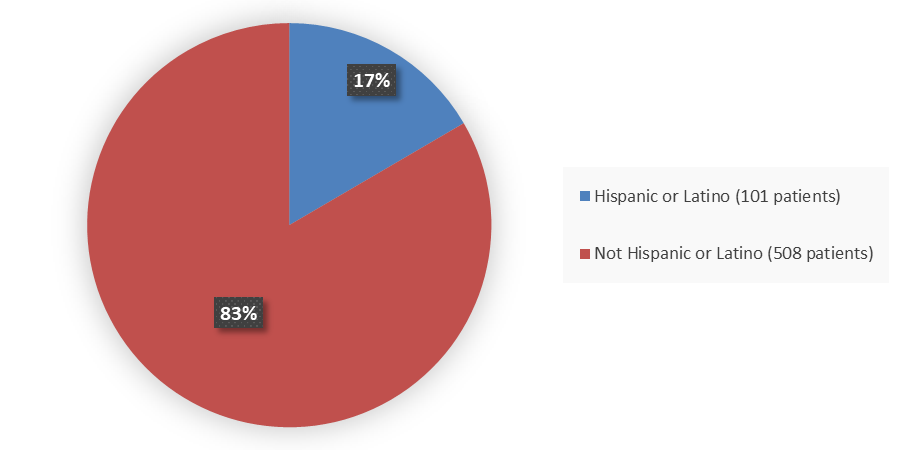
Not Hispanic or Latino |
154 (77) |
77 (75) |
185 (90) |
92 (91) |
508 (83) |
|
Hispanic or Latino |
47 (23) |
25 (25) |
20 (10) |
9 (9) |
101 (17) |
Source: Adapted from FDA Review
How were the trials designed?
The benefit and side effects of DAXXIFY were primarily evaluated in two clinical trials.
Both trials enrolled patients 18 to 75 years old with moderate to severe glabellar lines (wrinkles between the eyebrows). Patients received a single intramuscular injection of DAXXIFY or placebo at five sites within the muscles between the eyebrows.
The benefit of DAXXIFY was assessed by determining the proportion of patients achieving at least a 2-grade improvement of wrinkles between the eyebrows at maximum frown from baseline to Week 4. Improvement of wrinkles between the eyebrows at maximum frown was assessed independently by both the investigator and the patient using a 4-point grading scale (0 = none, 1 = mild, 2 = moderate, 3 = severe).
How were the trials designed?
The efficacy and safety of DAXXIFY were primarily evaluated in two randomized, double-blind, placebo-controlled trials.
Both trials enrolled patients ranging in age from 18 to 75 years old with moderate to severe glabellar facial lines at maximum frown. Patients were randomized to receive a single treatment with DAXXIFY or placebo intramuscularly. Patients received intramuscular injections (0.1 mL/injection site) of DAXXIFY or placebo at five sites, one in the procerus muscle and two in each corrugator supercilii muscle.
The primary efficacy endpoint was the proportion of patients achieving ≥2-grade improvement from baseline at maximum frown at Week 4. Improvement of glabellar line severity at maximum frown was assessed independently by both the investigator and the patient using a 4-point grading scale (0 = none, 1 = mild, 2 = moderate, 3 = severe).
DEMOGRAPHICS SNAPSHOT
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
What are the benefits of this drug?
In two trials, patients with moderate to severe wrinkles between the eyebrows had improvement of frown lines four weeks after treatment with DAXXIFY. In Trial GL-1, 74% of patients treated with DAXXIFY had temporary improvement of wrinkles between the eyebrows compared to 0% of patients in the group that received placebo. In Trial GL-2, 74% of patients treated with DAXXIFY had temporary improvement of wrinkles between the eyebrows compared to 0% of patients in the group that received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes efficacy results for the evaluated patients in the clinical trials. The primary endpoint (treatment success) was the proportion of patients achieving a score of 0 or 1 (none or mild) and ≥2-grade improvement from baseline at maximum frown, as assessed independently by both the investigator and the patient, using a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) at Week 4.
Table 2. Percentage of Subjects Achieving a Score of None or Mild and ≥2-Grade Improvement From Baseline on the Investigator and Subject Assessment of Glabellar Line Severity at Maximum Frown at Week 4
|
Parameter |
STUDY GL-1 |
STUDY GL-2 |
||||
|---|---|---|---|---|---|---|
|
DAXXIFY N=201 |
Placebo N=102 |
Treatment Difference |
DAXXIFY N=205 |
Placebo N=101 |
Treatment Difference |
|
|
Treatment success |
148 (74) |
0 (0) |
74 (68, 80) |
152 (74) |
0 (0) |
74 (68, 80) |
|
Individual component |
||||||
|
Investigator |
172 (86) |
1 (1) |
- |
187 (92) |
2 (2) |
- |
|
Subject |
152 (76) |
0 |
- |
156 (77) |
0 |
- |
Source: DAXXIFY Prescribing Information
Abbreviations: CI, confidence interval
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The number of males was small; therefore, differences in how well DAXXIFY worked in male patients compared to female patients could not be determined.
- Race: The number of patients of races other than White was small; therefore, differences in how well DAXXIFY worked among races could not be determined.
- Age: All subjects were 75 years of age or younger. The observed effect of DAXXIFY was larger among subjects 45 years of age or younger than in those 46 years of age or older. Because of limited data, this difference may be due to chance.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes efficacy results by sex, race, and age based on the proportion of patients achieving ≥2-grade improvement from baseline at maximum frown at Week 4.
Table 3. Primary Efficacy Endpoint by Subgroup (Studies GL-1 and GL-2; Subjects as Treated)
|
Subgroup |
Study GL-1 |
Study GL-2 |
||
|---|---|---|---|---|
|
DAXXIFY |
Placebo |
DAXXIFY |
Placebo |
|
|
Age (years) |
||||
|
18 to 45 |
50/58 (86) |
0/32 (0) |
50/63 (79) |
0/29 (0) |
|
46 to 55 |
45/68 (66) |
0/41 (0) |
70/91 (77) |
0/42 (0) |
|
56 to 75 |
53/75 (71) |
0/29 (0) |
32/51 (63) |
0/30 (0) |
|
Sex |
||||
|
Female |
134/174 (77) |
0/88 (0) |
137/184 (74) |
0/86 (0) |
|
Male |
14/27 (52) |
0/14 (0) |
15/21 (71) |
0/15 (0) |
|
Race |
||||
|
White |
126/173 (73) |
0/81 (0) |
134/181 (74) |
0/91 (0) |
|
Black or African American |
8/10 (80) |
0/8 (0) |
8/9 (89) |
0/3 (0) |
|
Asian |
6/7 (86) |
0/2 (0) |
7/11 (64) |
0/5 (0) |
|
Ethnicity |
||||
|
Not Hispanic or Latino |
109/154 (71) |
0/77 (0) |
136/185 (74) |
0/92 (0) |
|
Hispanic or Latino |
39/47 (83) |
0/25 (0) |
16/20 (80) |
0/9 (0) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
What are the possible side effects?
Botulinum toxin products may spread from the area of injection to other areas and cause serious life-threatening side effects including difficulty swallowing and difficulty breathing that could lead to death. Spread of botulinum toxin products to other areas can also cause loss of strength and muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, and loss of bladder control.
Other serious side effects of botulinum toxin products include severe allergic reactions and heart attack.
The most common side effects of DAXXIFY are headache, drooping eyelids, and weakness of facial muscles.
What are the possible side effects (results of trials used to assess safety)?
Table 4 summarizes adverse reactions in patients with moderate to severe glabellar lines in the clinical trials.
Table 4. Most Common Adverse Reactions ≥1% and More Frequent Than Placebo in Pooled Double-Blind, Placebo-Controlled Trials for Glabellar Lines
|
Adverse Reaction |
DAXXIFY |
Placebo |
|---|---|---|
|
Headache |
26 (6) |
4 (2) |
|
Eyelid ptosis |
9 (2) |
0 |
|
Facial paresis* |
5 (1) |
0 |
Source: DAXXIFY Prescribing Information
* Facial paresis, including facial asymmetry.
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The number of males was small; therefore, differences in the occurrence of side effects among male patients compared to female patients could not be determined.
- Race: The number of patients of races other than White was small; therefore, differences in in the occurrence of side effects among races could not be determined.
- Age: All subjects were 75 years of age or younger. The observed occurrence of side effects was larger among subjects 45 years of age or younger than in those 46 years of age or older. Because of limited data, this difference may be due to chance.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5 summarizes the occurrence of the most common adverse reaction, headache, by subgroup.
Table 5. Subgroup Analysis of Headache
|
Demographic Characteristic |
DAXXIFY |
Placebo |
|---|---|---|
|
Age (years) |
||
|
18 to 45 |
12/121 (10) |
2/61 (3) |
|
46 to 55 |
6/159 (4) |
2/83 (2) |
|
56 to 75 |
8/126 (6) |
0/59 (0) |
|
Sex |
||
|
Female |
22/358 (6) |
4/174 (2) |
|
Male |
4/48 (8) |
0/29 (0) |
|
Race |
||
|
White |
22/354 (6) |
3/172 (2) |
|
Black or African American |
1/19 (5) |
0/11 (0) |
|
Asian |
2/18 (11) |
0/7 (0) |
|
Ethnicity |
||
|
Not Hispanic or Latino |
22/339 (6) |
2/169 (1) |
|
Hispanic or Latino |
4/67 (6) |
2/34 (6) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.