Cardinal Health Recalls Monoject Disposable Syringes for Incompatibilities with Syringe Pumps
UPDATE: February 2, 2024
On February 2, 2024, Cardinal Health announced a recall for removal of certain Cardinal Health brand Monoject luer-lock and enteral syringes. See Do Not Use Certain Cardinal Health Monoject Luer-Lock and Enteral Syringes – FDA Safety Communication for the most current information on this recall.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Please be aware, this recall is a correction, not a product removal.
Recalled Product
- Product Names: Cardinal Health Monoject single use Luer lock syringes 1, 6, 12, 20, 35 and 60mL
- Product Codes: See Recall Database entries above
- Distribution Dates: June 1, 2023 to August 31, 2023
- Devices Recalled in the U.S.: 32,433,200
- Date Initiated by Firm: September 19, 2023
Device Use
Cardinal Health Monoject disposable syringes are used to inject fluid into or withdraw fluids from the body. When used with syringe pumps, the Monoject disposable syringes are loaded with fluid or medications and placed into the pump.
Syringe pumps deliver solutions such as fluids, medications, and blood products to patients.
Reason for Recall
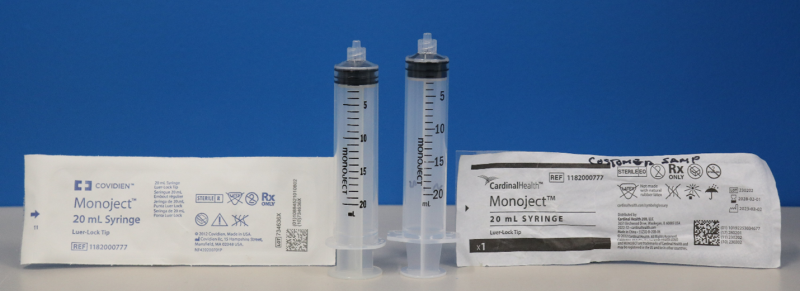
In June 2023, Cardinal Health began distributing Monoject syringes branded as “Cardinal Health Monoject syringes.” These new syringes differ from the previously branded “Covidien Monoject syringes” as they have different dimensions.
As pictured above, the Covidien Monoject syringe on the left has different dimensions than the Cardinal Health Monoject syringe on the right.
The affected Cardinal Health Monoject syringes (seen on the right in the figure above) should not be used with syringe pumps. The dimensional changes made to the affected Cardinal Health Monoject syringes when used with syringe pumps may result in pump performance issues such as overdose, underdose, delay in therapy, and delays in occlusion alarms.
Cardinal Health has received 15 reports of delayed therapy due to syringe infusion pumps not recognizing syringes, and 13 reports of inaccurate volume/rate dispensing, including some injuries. Cardinal Health has not received any reports of patient death.
Who May be Affected
- People who receive care with Cardinal Health Monoject Disposable Syringes used with syringe pumps.
- Health care providers who use Cardinal Health Monoject Disposable Syringes with syringe pumps.
What to Do
- Do not use affected Cardinal Health Monoject syringes with syringe pumps. Refer to the table below for a list of affected products.
- You may continue to use Covidien Monoject syringes with syringe pumps.
On September 20, 2023, Cardinal Health sent all affected customers an Urgent Medical Device Product Correction Letter.
The letter requested customers to:
- Review your inventory for the affected product codes and lots. Product codes and lots are shown in above listed table.
- Communicate with all personnel that utilize the Cardinal Health Monoject Luer-Lock Tip syringes (1, 6, 12, 20, 35 and 60 mL) that they should not be used with syringe infusion pumps.
- Post a copy of this notification in your storeroom where the product is stored.
- Notify any customers to whom you may have distributed/forwarded affected product to or will send the product on to about this medical device product correction and share a copy of this notice.
- Return the provided acknowledgment form via fax to 614-652-9648 or email to GMB-FieldCorrectiveAction@cardinalhealth.com, whether you have affected product or not.
Contact Information
Customers in the U.S. with questions about this recall should contact the Cardinal Health market action team at 1-800-292-9332 or email GMB-FieldCorrectiveAction@cardinalhealth.com.
Full List of Affected Devices
The names of the devices and associated product codes being recalled:
| Product Code | Product Description | UDI | Lot Numbers |
|---|---|---|---|
| 1180100777 | Monoject 1 mL Tuberculin Syringe Luer-Lock Tip Soft Pack | 10192253034530 - each 20192253034537 - box 50192253034538 - case |
221201, 221202, 221203, 230201, 230202, 230203, 230204, 230205, 230601 |
| 1180600777 | Monoject 6 mL Syringe Luer-Lock Tip Soft Pack | 10192253034608- each 20192253034605- box 50192253034606- case |
221201, 221202, 221203, 221204, 221205, 230201, 230202, 230203, 230204, 230205, 230206, 230207 |
| 1181200777T | Monoject 12 mL Syringe Luer-Lock Tip Soft Pack | 10192253025811-each 20192253025818-box 50192253025819-case |
221101, 221102, 221103, 21104 |
| 1182000777 | Monoject 20 mL Syringe Luer-Lock Tip Soft Pack | 10192253034677-each 20192253034674-box 50192253034675-case |
221201, 221202, 221203, 221204, 221205, 230201, 230202, 230203, 230204, 230205, 230206 |
| 1183500777 | Monoject 35 mL Syringe Luer-Lock Tip Soft Pack | 10192253034691-each 20192253034698-box 50192253034699-case |
221201, 230201, 230601, 230602 |
| 1186000777T | Monoject 60 mL Syringe Luer-Lock Tip Soft Pack | 10192253025835-each 20192253025832-box 50192253025833-case |
221101, 230601 |
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.