Focus Area: Novel Foods and Food Ingredients
Importance to FDA
Innovations in food ingredients and food production technologies provide consumers additional food choices and may improve public health, food productivity, and food security. Some of these ingredients and foods are new to the supply chain, and additional scientific information related to these new food ingredients is valuable. FDA conducts research about the safety, regulation, labeling, and use of these products to best protect consumers and make regulatory recommendations and decisions.
Examples
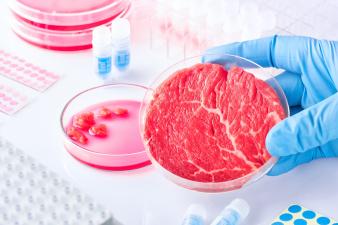
- FDA is working in cooperation with the U.S. Department of Agriculture to create a clear regulatory pathway for industry efforts to produce foods made from cultured cells of animals. In addition to evaluating the safety of new food additives or ingredients derived from this technology to ensure safety, FDA is considering review of compositional data for a variety of nutritive and non-nutritive constituents from a range of conventionally-sourced animal tissues to inform assessments of both identity and safety regarding products of this technology as compared to conventional foods. Availability of this information could potentially help support use of animal cell culture technology by industry to expand the food choices available to consumers. Availability of this information could potentially help support use of animal cell culture technology by industry to expand the food choices available to consumers.
- Scientific advancements in genome editing have led to the ability to more efficiently and precisely alter the genomes of food-producing organisms to provide desired traits. Such traits might otherwise only be achievable by laborious plant cross-breeding techniques, if at all. FDA has evaluated the safety of food from more than 180 varieties of genetically engineered plants and food from a genome-edited plant variety. If FDA’s evaluation identifies safety or regulatory questions, the agency will request further information from developers to resolve them.
- Developed and validated a method to isolate Salmonella sp. from the increasing variety of plant-based meat alternatives on the commercial market, using Salmonella serotypes (S. enteritidis or S. agona) previously linked to plant-based protein ingredients. This project enhanced the agency’s readiness to respond to potential future outbreaks associated with these novel food matrices.
| Minority Health and Health Equity | Women’s Health | Maternal Health | Pediatric Health |
| Oncology | Rare Diseases | One Health Initiative |
Research Capabilities, Tools, and Resources
| Research Management and Collaborations | Technology Transfer and Public-Private Partnerships | Physical Standards and Reference Materials | Intramural Grant Programs | Extramural Funding Mechanisms |
Scientific Education, Training, and Communication
| Fellowship and Training Opportunities | Professional Development and Continuing Education | Communication and External Meetings |
Infrastructure
| Facilities and Shared Resources | Safety and Compliance |
| Office of the Chief Scientist | Contact Us: FARS@fda.hhs.gov |