Drug Trials Snapshots: DAYBUE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the DAYBUE Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
DAYBUE (trofinetide)
day-BYOO
ACADIA PHARMACEUTICALS INC.
Original Approval date: March 12, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DAYBUE is a prescription medicine that is used to treat Rett syndrome in adults and children 2 years of age and older.
How is this drug used?
DAYBUE is a liquid that may be taken by mouth or given through a surgically placed feeding tube called a gastrostomy (G) tube, twice daily. If DAYBUE is given through a gastrojejunal (GJ) tube, the G-port must be used.
Who participated in the clinical trials?
The FDA approved DAYBUE based on evidence from a clinical trial of 187 patients with Rett syndrome. The trial was conducted at 21 sites in the United States.
How were the trials designed?
DAYBUE was evaluated in one clinical trial of 187 patients with Rett syndrome. Study 1 was a randomized, multicenter, double-blind, placebo-controlled trial in patients with typical Rett syndrome. Patients 5 to 20 years of age were randomized 1:1 to receive DAYBUE at the recommended dose or placebo. The coprimary efficacy endpoints were the change from baseline in Rett Syndrome Behavior Questionnaire (RSBQ) and the Clinical Global Impression-Improvement (CGI-I) at 12 weeks.
How were the trials designed?
The efficacy of DAYBUE for the treatment of Rett syndrome was established in a 12-week randomized, double-blind, placebo-controlled study in patients with Rett syndrome 5 to 20 years of age (Study 1; NCT04181723).
Patients (N=187) had a diagnosis of typical Rett syndrome according to the Rett Syndrome Diagnostic Criteria with a documented disease-causing mutation in the MECP2 gene. Patients were randomized to receive DAYBUE (N=93) or matching placebo (N=94) for 12 weeks. The DAYBUE dosage was based on patient weight to achieve similar exposure in all patients (Table 1).
Table 1. Dosing of DAYBUE in Study 1
|
Patient Weight |
DAYBUE Dosage |
DAYBUE Volume |
|---|---|---|
|
12 kg to <20 kg |
6000 mg twice daily |
30 mL twice daily |
|
20 kg to <35 kg |
8000 mg twice daily |
40 mL twice daily |
|
35 kg to <50 kg |
10000 mg twice daily |
50 mL twice daily |
|
≥50 kg |
12000 mg twice daily |
60 mL twice daily |
Source: DAYBUE Prescribing Information
DEMOGRAPHICS SNAPSHOT
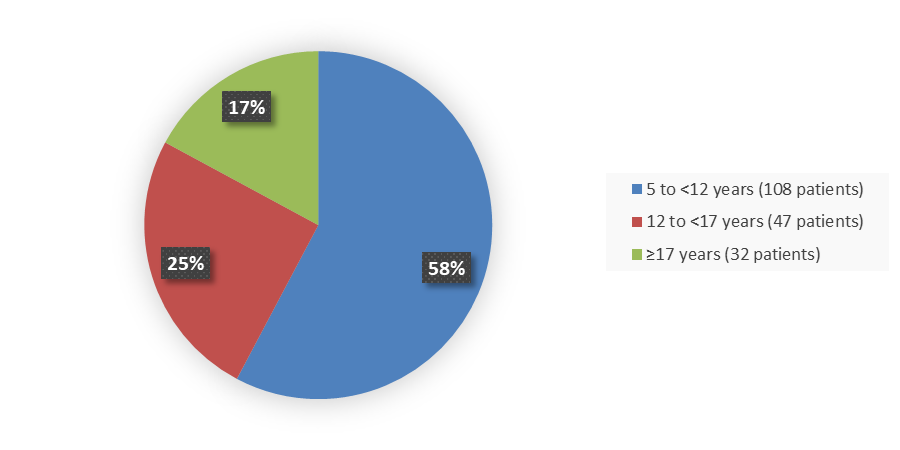
Figure 1 summarizes how many patients in each age group were enrolled in Study 1.
Figure 1. Baseline Demographics by Age
Source: Adapted from FDA Review
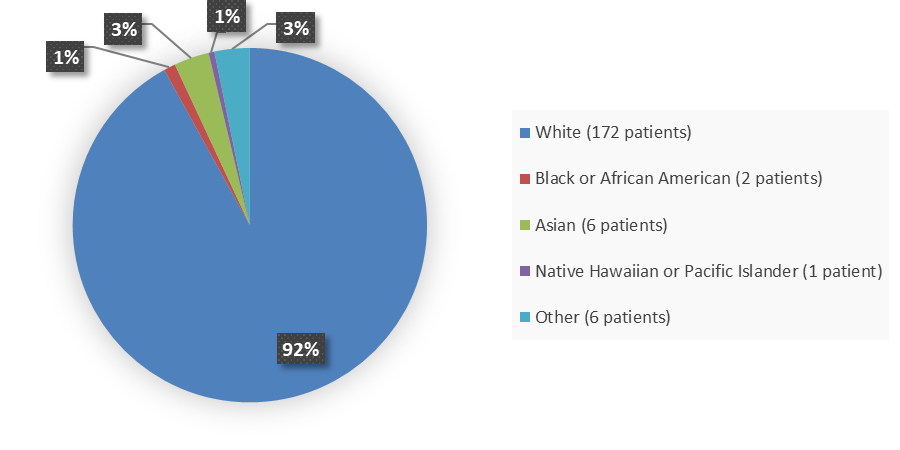
Figure 2 summarizes the percentage of patients by race enrolled in Study 1.
Figure 2. Demographics by Race
Source: Adapted from FDA Review
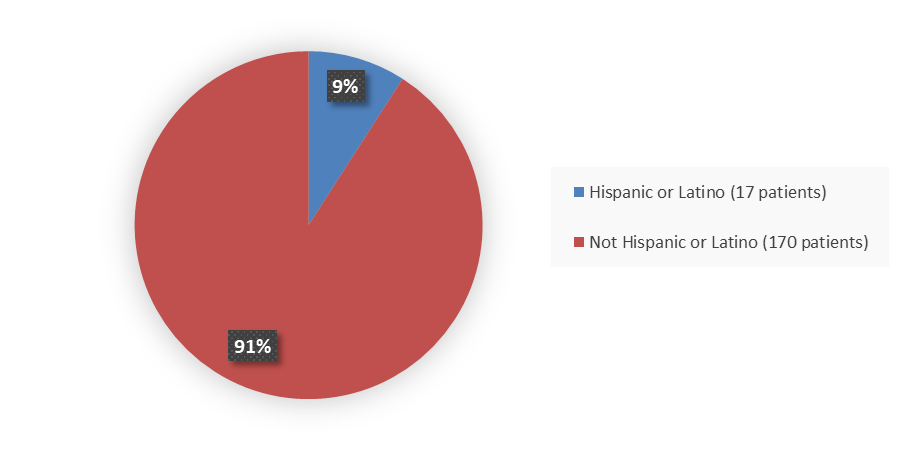
Figure 3 summarizes the percentage of patients by ethnicity enrolled in Study 1.
Figure 3. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 2. Demographics - Randomized Analysis Set
|
Demographic |
DAYBUE |
Placebo |
Total |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Female |
93 (100.0) |
94 (100.0) |
187 (100.0) |
|
Age, years |
|||
|
Mean (SE) |
11.0 (0.49) |
10.9 (0.47) |
10.9 (0.34) |
|
SD |
4.69 |
4.57 |
4.62 |
|
Median |
10.0 |
10.0 |
10.0 |
|
Min, max |
5, 20 |
5, 20 |
5, 20 |
|
Age categories, years, n (%) |
|||
|
5 to <12 |
53 (57.0) |
55 (58.5) |
108 (57.8) |
|
12 to <17 |
23 (24.7) |
24 (25.5) |
47 (25.1) |
|
≥17 |
17 (18.3) |
15 (16.0) |
32 (17.1) |
|
Race, n (%) |
|||
|
White |
82 (88.2) |
90 (95.7) |
172 (92.0) |
|
Black or African American |
1 (1.1) |
1 (1.1) |
2 (1.1) |
|
Asian |
5 (5.4) |
1 (1.1) |
6 (3.2) |
|
Native Hawaiian or other Pacific Islander |
1 (1.1) |
0 |
1 (<1) |
|
Other |
4 (4.3) |
2 (2.1) |
6 (3.2) |
|
Race group |
|||
|
Other |
11 (11.8) |
4 (4.3) |
15 (8.0) |
|
White |
82 (88.2) |
90 (95.7) |
172 (92.0) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
7 (7.5) |
10 (10.6) |
17 (9.1) |
|
Not Hispanic or Latino |
86 (92.5) |
84 (89.4) |
170 (90.9) |
|
Weight (kg) |
|||
|
Mean (SE) |
30.5 (1.31) |
29.2 (1.07) |
29.9 (0.84) |
|
SD |
12.61 |
10.37 |
11.53 |
|
Median |
29.6 |
27.3 |
28.1 |
|
Min, max |
13, 78 |
13, 57 |
13, 78 |
|
BMI (kg/m2) |
|||
|
Mean (SE) |
17.7 (0.46) |
17.2 (0.36) |
17.4 (0.29) |
|
SD |
4.43 |
3.46 |
3.97 |
|
Median |
16.8 |
16.6 |
16.7 |
|
Min, max |
10, 34 |
12, 28 |
10, 34 |
|
Missing |
1 |
3 |
4 |
Source: Adapted from FDA Review
Abbreviations: BMI, body mass index; Max, maximum; Min, minimum; SD, standard deviation; SE, standard error
What are the benefits of this drug?
In the clinical trial, caregivers rated patients’ symptoms on the RSBQ. The symptoms of patients with Rett syndrome who received DAYBUE for 12 weeks were less severe than those who received placebo. In addition, the health care providers for these patients rated them as having mildly improved compared to those who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The two coprimary endpoints in Study 1 were the RSBQ and the CGI-I. The RSBQ is a 90-point scale that measures caregiver impressions of the severity and frequency of various Rett syndrome symptoms, with lower scores meaning less frequent and less severe symptoms. The CGI-I is a 7-point scale that measures the clinician’s impression of improvement at the end of the trial, where a score of 1 indicates very much improved, 4 indicates no change, and 7 indicates very much worse.
Table 3. Summary of Study 1 Efficacy in Patients at Month 3 of Treatment
|
Parameter |
DAYBUE |
Placebo |
|---|---|---|
|
RSBQ |
||
|
Mean baseline score (SE) |
43.7 (1.21) |
44.5 (1.26) |
|
Mean Week 12 score (SE) |
39.9 (1.38) |
42.8 (1.42) |
|
LS mean change from baseline to Week 12 (SE) |
-4.9 (0.94) |
-1.7 (0.90) |
|
p-valuea |
0.018 |
|
|
CGI-I |
||
|
Mean Week 12 score (SE) |
3.5 (0.08) |
3.8 (0.06) |
|
p-valuea |
0.003 |
|
Source: Adapted from DAYBUE Prescribing Information
a Difference in LS mean from the mixed-effect model for repeated measure analysis
Abbreviations: CGI-I, Clinical Global Impression - Improvement; LS mean, least-squares mean; RSBQ, Rett Syndrome Behavior Questionnaire; SE, standard error
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DAYBUE was only tested in females because the number of patients with Rett syndrome that are male is very small. However, DAYBUE is approved for male and female patients diagnosed with Rett syndrome.
- Race: The number of patients of races other than white was small; therefore, differences in response to DAYBUE among races could not be determined.
- Age: DAYBUE was tested in patients with Rett syndrome ages 2 through 20 and worked equally well in all groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The differences in response to DAYBUE by age group in Study 1 is displayed in Figure 4.
Figure 4. Forest Plot of LSM Treatment Difference With 95% CI (MMRM) for Coprimary Endpoints by Age Group
Source: Adapted from FDA Review
Abbreviations: CGI-I, Clinical Global Impression - Improvement; CI, confidence interval; LSM, least squares mean; MMRM, mixed-effects model for repeated measures; RSBQ, Rett Syndrome Behavior Questionnaire
What are the possible side effects?
DAYBUE may cause side effects including diarrhea and weight loss.
The most common side effects in studies with DAYBUE included diarrhea and vomiting.
What are the possible side effects (results of trials used to assess safety)?
In Study 1, 82% of patients receiving DAYBUE experienced diarrhea. Also, in long-term studies of DAYBUE, 49% of patients continued to have diarrhea even after DAYBUE doses were interrupted or reduced and antidiarrheal mediations were started.
In Study 1, 12% of patients receiving DAYBUE experienced weight loss greater than 7% from baseline compared to 4% of patients receiving placebo.
Common side effects from DAYBUE are listed in Table 4.
Table 4. Side Effects in at Least 5% of Patients Treated With DAYBUE and at Least 2% Greater Than Placebo in Study 1
|
Adverse Reaction |
DAYBUE |
Placebo |
|---|---|---|
|
Diarrhea |
82 |
20 |
|
Vomiting |
29 |
12 |
|
Fever |
9 |
4 |
|
Seizure |
9 |
6 |
|
Anxiety |
8 |
1 |
|
Decreased appetite |
8 |
2 |
|
Fatigue |
8 |
2 |
|
Nasopharyngitis |
5 |
1 |
Source: DAYBUE Prescribing Information
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: DAYBUE was only tested in females because very few patients with Rett syndrome are male. It is unlikely that any DAYBUE side effects are different between sexes.
- Race: The number of patients in the clinical trial was small, and the majority of patients were White; therefore, differences in side effects to DAYBUE among races could not be determined.
- Age: DAYBUE was tested in patients with Rett syndrome ages 2 through 20 and similar side effects occurred across age groups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
After Study 1, patients could enroll in a long-term study of DAYBUE in which all patients received DAYBUE.
Table 5. Side Effects to DAYBUE in Study 1 and Open Label Long Term Studies by Age Group
|
Parameter |
5 to <12 Years |
12 to <17 Years |
≥17 Years |
Overall |
|---|---|---|---|---|
|
Any side effect |
99 (95.2) |
41 (95.3) |
30 (96.8) |
170 (95.5) |
|
Any serious side effect |
20 (19.2) |
8 (18.6) |
3 (9.7) |
31 (17.4) |
|
Any side effect leading to patients stopping DAYBUE |
45 (43.3) |
14 (32.6) |
13 (41.9) |
72 (40.4) |
Source: Adapted from FDA Review
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.