Drug Trials Snapshots: JAYPIRCA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the JAYPIRCA Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
JAYPIRCA (pirtobrutinib)
(JAY-PIHRKAA)
Loxo Oncology Inc.
Approval date: January 27, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
JAYPIRCA is a Bruton tyrosine kinase (BTK) inhibitor that is used to treat a form of cancer called mantle cell lymphoma (MCL). It is to be used in adult patients whose cancer came back after or did not respond to at least two prior treatment regimens which included a BTK inhibitor.
How is this drug used?
JAYPIRCA is a tablet that is taken by mouth once per day.
Who participated in the clinical trials?
The FDA approved JAYPIRCA based on evidence from one clinical trial of patients with hematologic malignancies. The efficacy is JAYPIRCA is based on 120 patients with MCL and the safety of JAYPIRCA is primarily based on 128 patients with MCL and supported by data in 583 patients with pooled hematologic malignancies. The trial was conducted at 49 sites in 10 countries in the United States, Europe, Australia, and Asia. The same trial was used to assess safety and efficacy.
How were the trials designed?
One clinical trial provided data for the approval of JAYPIRCA. The trial enrolled patients with hematologic malignancies, including MCL. All patients in the trial received JAYPIRCA until their disease progressed or the side effects became too toxic. The benefit of JAYPIRCA was measured by the proportion of patients that achieved a clinically relevant improvement in their disease (overall response rate; ORR).
How were the trials designed?
The efficacy and safety of JAYPIRCA was evaluated in one clinical trial in with hematologic malignancies. The efficacy and safety populations included patients with MCL whose disease came back after or did not respond to at least two prior treatment regimens, including a BTK inhibitor, and received JAYPIRCA at the recommended dose; the efficacy population included 120 patients and the safety population included 128 patients. The primary endpoint in the trial was ORR, defined as the proportion of patients who achieved complete response or partial response, as determined by an independent review committee based on the 2014 Lugano Criteria.
DEMOGRAPHICS SNAPSHOT
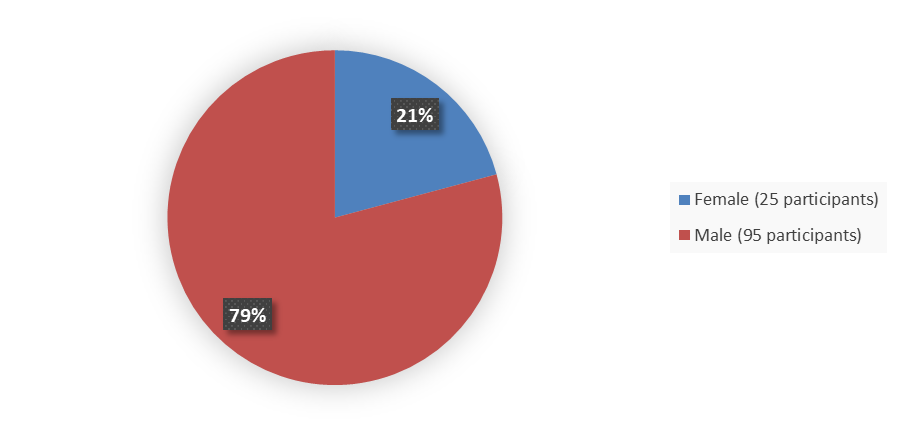
Figure 1 summarizes how many males and females were enrolled in the clinical trial used to evaluate the efficacy of JAYPIRCA.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
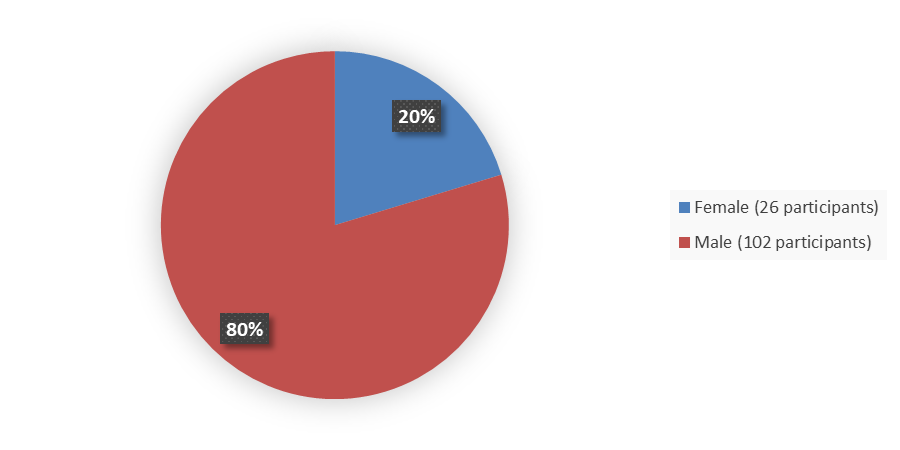
Figure 2 summarizes how many males and females were enrolled in the clinical trial used to evaluate the side effects of JAYPIRCA.
Figure 2. Baseline Demographics by Sex, Safety Population
Source: Adapted from FDA Review
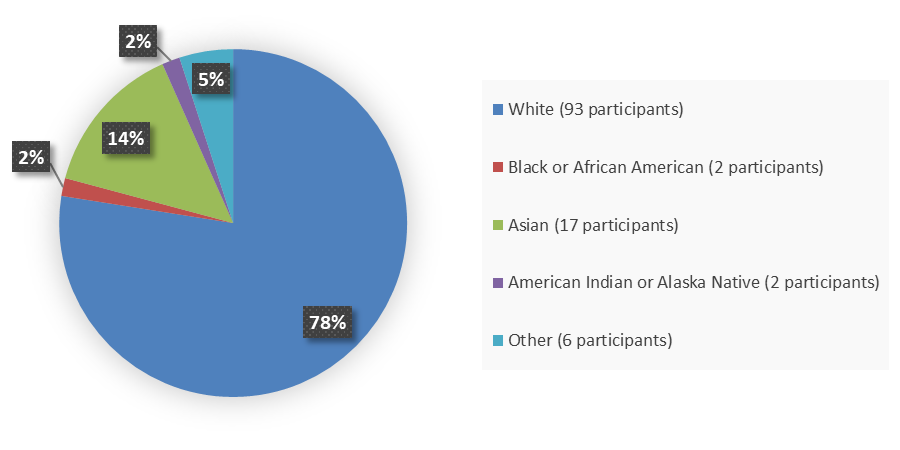
Figure 3 summarizes how many patients by race were in the trial used to evaluate the efficacy of JAYPIRCA.
Figure 3. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
*Other includes Mauritian (n=1), not specified (n=2), unknown (n=1), patient reported other (n=1), not reported due to country policy (n=1)
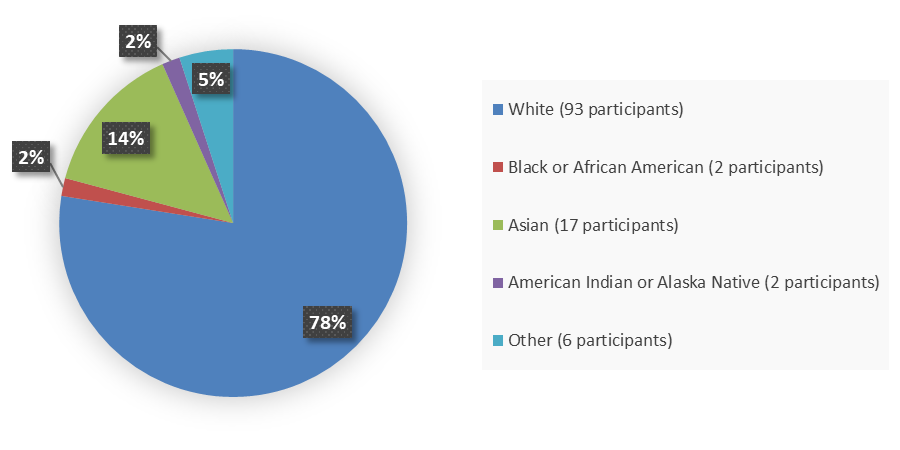
Figure 4 summarizes the percentage of patients by race who were enrolled in the clinical trial used to evaluate the side effects of JAYPIRCA.
Figure 4. Baseline Demographics by Race, Safety Population
Source: Adapted from FDA Review
*Other includes not specified (n=2), unknown (n=1), patient reported other (n=1), not reported due to country policy (n=1)
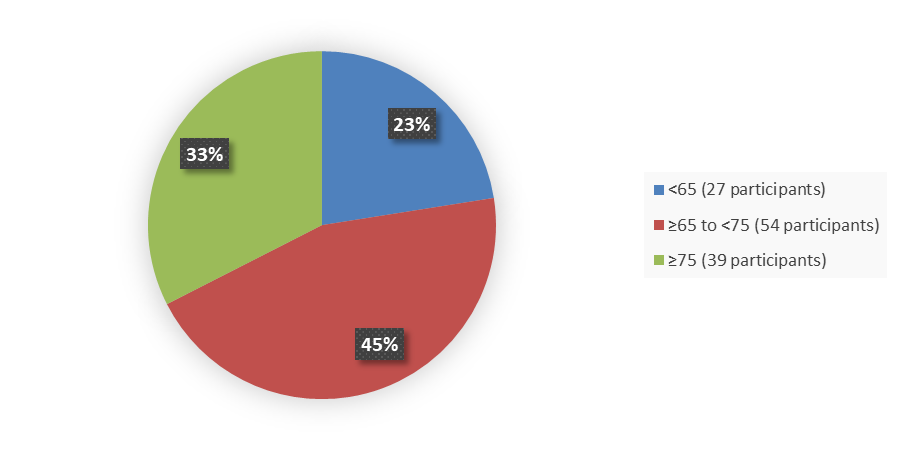
Figure 5 summarizes how many patients by age were in the trial used to evaluate the efficacy of JAYPIRCA.
Figure 5. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
Figure 6 summarizes the percentage of patients by age who were enrolled in the clinical trial used to evaluate the side effects of JAYPIRCA.
Figure 6. Baseline Demographics by Age, Safety Population
Source: Adapted from FDA Review
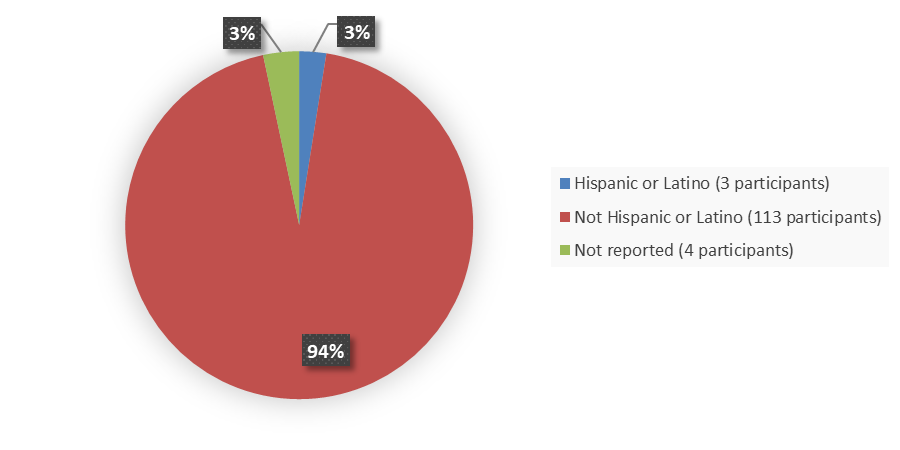
Figure 7 summarizes how many patients by ethnicity were in the trial used to evaluate the efficacy of JAYPIRCA.
Figure 7. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
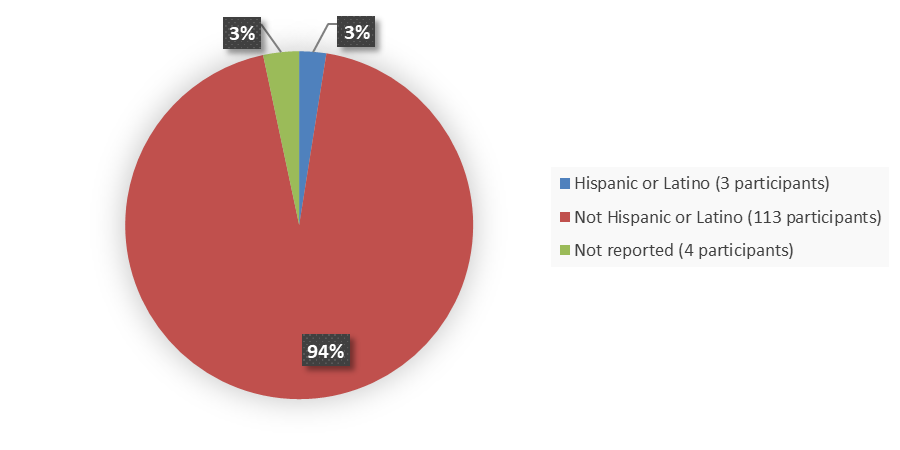
Figure 8 summarizes the percentage of patients by ethnicity who were enrolled in the clinical trial used to evaluate the side effects of JAYPIRCA.
Figure 8. Baseline Demographics by Ethnicity, Safety Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Demographics of Patients in the Trial
| Demographic Parameter | JAYPIRCA Efficacy Population N=120 n (%) |
JAYPIRCA Safety Population N=128 n (%) |
|---|---|---|
| Sex | ||
| Male | 95 (79) | 102 (80) |
| Female | 25 (21) | 26 (20) |
| Race | ||
| White | 93 (78) | 100 (78) |
| Asian | 17 (14) | 18 (14) |
| Black or African American | 2 (1.7) | 3 (2.3) |
| American Indian or Alaska Native | 2 (1.7) | 2 (1.6) |
| Other | 6 (5)a | 5 (3.9)b |
| Age, years | ||
| <65 | 27 (23) | 30 (23) |
| ≥65 to <75 | 54 (45) | 55 (43) |
| ≥75 | 39 (33) | 43 (34) |
| Ethnicity | ||
| Hispanic or Latino | 3 (2.5) | 3 (2.3) |
| Not Hispanic or Latino | 113 (94) | 121 (95) |
| Not reported | 4 (3.3) | 4 (3.1) |
| Region | ||
| United States | 73 (61) | 81 (63) |
| Rest of the world | 47 (39) | 47 (37) |
Source: Adapted from FDA Review
Abbreviation: CI, confidence interval; NE, not estimable
What are the benefits of this drug?
In the trial, 60 out of 120 patients (50%) treated with JAYPIRCA experienced an improvement in their MCL. For 35% of patients who responded, the improvement lasted at least 6 months; for 15% of patients who responded, the improvement lasted at least 12 months.
JAYPIRCA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of JAYPIRCA was evaluated by ORR, as determined by an independent review committee based on 2014 Lugano Criteria (Table 2).
Table 2. Efficacy Results
| Efficacy Parameter | JAYPIRCA N=120 |
|---|---|
| Response rate, n (%) | |
| Overall response rate | 60 (50) |
| 95% CI | 41, 59 |
| Complete response | 15 (13) |
| Partial response | 45 (38) |
| Stable disease | 26 (22) |
| Progressive disease | 28 (23) |
| Not evaluable | 6 (5) |
| Duration of response | |
| Median, months (95% CI) | 8.3 (5.7, NE) |
Source: Adapted from FDA Review
Abbreviation: CI, confidence interval; NE, not estimable
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: Most patients were male. Differences in effectiveness among sexes could not be determined because of the small numbers of female patients.
- Race: Most patients were White. Differences in effectiveness among races could not be determined because of the small numbers of patients in other races.
- Age: In the efficacy population, most patients were older than 65 years of age. Differences in effectiveness based on age could not be determined because of the small numbers of patients younger than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes subgroup analysis of ORR. Because the number of patients in each subgroup was relatively small, the subgroup analyses are considered exploratory.
Table 3. Subgroup Analysis of Overall Response Rate
| Subgroup | n/Ns (%) | 95% CI |
|---|---|---|
| All patients | 60/120 (50) | 41, 59 |
| Sex | ||
| Male | 47/95 (49) | 39, 60 |
| Female | 13/25 (52) | 31, 72 |
| Race | ||
| White | 46/93 (49) | 39, 60 |
| Asian | 9/17 (53) | 28, 77 |
| Othera | 5/10 (50) | 19, 81 |
| Age, years | ||
| <65 | 14/27 (52) | 32, 71 |
| ≥65 to <75 | 23/54 (43) | 29, 57 |
| ≥75 | 23/39 (59) | 42, 74 |
| Ethnicity | ||
| Not Hispanic or Latino | 56/113 (50) | 40, 59 |
| Hispanic or Latino | 1/3 (33) | 0.8, 91 |
| Unknown | ¾ (75) | 19, 99 |
Source: Adapted from FDA Review
a Other includes Other (n=6), Black or African American (n=2), and American Indian or Alaska Native (n=2)
Abbreviations: CI, confidence interval; n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
What are the possible side effects?
JAYPIRCA may cause side effects that are serious or life-threatening including infections, bleeding, decreased blood counts, heart rhythm problems, and second primary cancers. The most common side effects of JAYPIRCA are tiredness; muscle, joint, and bone pain; diarrhea; swelling; shortness of breath; pneumonia; and bruising.
What are the possible side effects (results of trials used to assess safety)?
Table 4 and Table 5summarizes the adverse reactions in the 128 patients (safety population) with MCL who received JAYPIRCA at the recommended dose in the clinical trial.
Table 4. Adverse Reactions (≥10%) in Patients With MCL Who Received JAYPIRCA
| Adverse Reactionsa | JAYPIRCA N=128 |
|
|---|---|---|
| All Grades (%) | Grade 3 or 4 (%) | |
| General disorders | ||
| Fatigue | 29 | 1.6 |
| Edema | 18 | 0.8 |
| Fever | 13 | - |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal pain | 27 | 3.9 |
| Arthritis or arthralgia | 12 | 0.8 |
| Gastrointestinal disorders | ||
| Diarrhea | 19 | - |
| Constipation | 13 | - |
| Abdominal pain | 11 | 0.8 |
| Nausea | 11 | - |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dyspnea | 17 | 2.3 |
| Cough | 14 | - |
| Injury | ||
| Bruising | 16 | - |
| Infections | ||
| Pneumonia | 16b | 14 |
| Upper respiratory tract infections | 10 | 0.8 |
| Nervous system disorders | ||
| Peripheral neuropathy | 14 | 0.8 |
| Dizziness | 10 | - |
| Skin and subcutaneous disorders | ||
| Rash | 14 | - |
| Vascular disorders | ||
| Hemorrhage | 11c | 3.1 |
Source: JAYPIRCA Prescribing Information
a Each term listed includes other related terms
b Includes one fatality from COVID-19 pneumonia
c Includes one fatality from hemorrhage
Abbreviations: MCL, mantle cell lymphoma
Table 5. Select Laboratory Abnormalities (≥10%) That Worsened From Baseline in Patients With MCL Who Received JAYPIRCA
| Laboratory Abnormality | JAYPIRCAa N=128 |
|
|---|---|---|
| All Grades (%) | Grade 3 or 4 (%) | |
| Hematology | ||
| Hemoglobin decreased | 42 | 9 |
| Platelet count decreased | 39 | 14 |
| Neutrophil count decreased | 36 | 16 |
| Lymphocyte count decreased | 32 | 15 |
| Chemistry | ||
| Creatinine increased | 30 | 1.6 |
| Calcium decreased | 19 | 1.6 |
| AST increased | 17 | 1.6 |
| Potassium decreased | 13 | 1.6 |
| Sodium decreased | 13 | - |
| Lipase increased | 12 | 4.4 |
| Alkaline phosphatase increased | 11 | - |
| ALT increased | 11 | 1.6 |
| Potassium increased | 11 | 0.8 |
Source: JAYPIRCA Prescribing Information
a The denominator used to calculate the rate varied from 90 to 127 based on the number of patients with a baseline value and at least one post-treatment value
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; MCL, mantle cell lymphoma
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males or females.
- Race: Most patients were White. Differences in side effects among races could not be determined because of the small numbers of patients in other races.
- Age: The occurrence of side effects was higher in patients 65 years and older.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 7 summarize the adverse reactions by subgroups in the 128 patients with MCL who received JAYPIRCA and the 583 patients with all hematologic malignancies who received JAYPIRCA at the recommended dose in the clinical trial. There were too few patients in the subgroups by race to determine differences in side effects by race.
Table 7. Overview of Side Effects by Sex, Race, and Age in Trial LOXO-BTK-18001, Safety Population, Entire Study Period
| Mantle Cell Lymphoma N=128 |
All Hematologic Malignancies N=583 |
|||||
|---|---|---|---|---|---|---|
| Characteristic | All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
| Sex, n (%) | ||||||
| Female | 26 (20.3) | 25/26 (96.2) | 13/26 (50.0) | 196 (33.6) | 185/196 (94.4) | 102/196 (52.0) |
| Male | 102 (79.7) | 89/102 (87.3) | 50/102 (49.0) | 387 (66.4) | 359/387 (92.8) | 190/387 (49.1) |
| Race, n (%) | ||||||
| American Indian or Alaska Native | 2 (1.6) | 2/2 (100) | 2/2 (100) | 2 (0.3) | 2/2 (100) | 2/2 (100) |
| Asian | 18 (14.1) | 14/18 (77.8) | 8/18 (44.4) | 42 (7.2) | 33/42 (78.6) | 19/42 (45.2) |
| Black or African American | 3 (2.3) | 2/3 (66.7) | 1/3 (33.3) | 17 (2.9) | 16/17 (94.1) | 9/17 (52.9) |
| Native Hawaiian or Other Pacific Islander | 0 (0) | 0/0 (NA) | 0/0 (NA) | 3 (0.5) | 3/3 (100) | 1/3 (33.3) |
| Other | 5 (3.9) | 2/5 (40.0) | 1/5 (20.0) | 21 (3.6) | 17/21 (81.0) | 7/21 (33.3) |
| White | 100 (78.1) | 94/100 (94.0) | 51/100 (51.0) | 498 (85.4) | 473/498 (95.0) | 254/498 (51.0) |
| Age group, years, n (%) | ||||||
| < 65 years | 30 (23.4) | 28/30 (93.3) | 13/30 (43.3) | 191 (32.8) | 175/191 (91.6) | 78/191 (40.8) |
| >= 65 years | 98 (76.6) | 86/98 (87.8) | 50/98 (51.0) | 392 (67.2) | 369/392 (94.1) | 214/392 (54.6) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 3 (2.3) | 2/3 (66.7) | 1/3 (33.3) | 25 (4.3) | 21/25 (84.0) | 11/25 (44.0) |
| Not Hispanic or Latino | 121 (94.5) | 109/121 (90.1) | 60/121 (49.6) | 531 (91.1) | 498/531 (93.8) | 272/531 (51.2) |
| Unknown | 4 (3.1) | 3/4 (75.0) | 2/4 (50.0) | 27 (4.6) | 25/27 (92.6) | 9/27 (33.3) |
Source: adae.xpt (eCTD seq 0021); FDA reviewer’s analysis
Software: R
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; Ns, total number of patients in each category
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.