Drug Trials Snapshots: OGSIVEO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the OGSIVEO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
OGSIVEO (nirogacestat)
og-SIH-vee-oh
SpringWorks Therapeutics, Inc
Original Approval date: November 27, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OGSIVEO is a gamma secretase inhibitor indicated for adult patients with progressing desmoid tumors who require systemic treatment.
How is this drug used?
OGSIVEO is a tablet that is taken as three 50 mg tablets (total dose of 150 mg) orally two times a day.
Who participated in the clinical trials?
The FDA approved OGSIVEO based on efficacy and safety evidence from one pivotal clinical trial (DeFi trial, NCT03785964) in patients with progressing desmoid tumors who required systemic treatment. A total of 142 patients were randomized, of whom 141 received at least one dose of trial treatment (OGSIVEO or placebo). Patients were randomized at 37 sites in 7 of countries including Belgium, Canada, Germany, Italy, Netherlands, United Kingdom, and the United States.
How were the trials designed?
The benefits and side effects of OSIVEO were evaluated in the DeFi trial in adults with progressing desmoid tumors who required a medicine by mouth or injection (systemic therapy). Eligible patients were randomized to receive either OGSIVEO or placebo. Patients and healthcare practitioners did not know which treatment was being administered. Treatment was administered continuously in 28-day cycles until the disease worsened or the patient experienced unacceptable side effects. The trial measured the length of time before tumor growth, disease worsening, or death after treatment (progression-free survival [PFS]) in each group.
How were the trials designed?
The benefit and side effects of OGSIVEO were evaluated in one clinical trial in adult patients with progressing desmoid tumors who required a medicine by mouth or injection (systemic therapy).
In the trial, patients were randomized to receive OGSIVEO or placebo. The treatment continued until disease progression or the side effects became too toxic.
The benefit of OGSIVEO was evaluated by measuring the length of time before tumor growth, disease worsening, or death after treatment (PFS) between patients who received OGSIVEO or placebo.
DEMOGRAPHICS SNAPSHOT
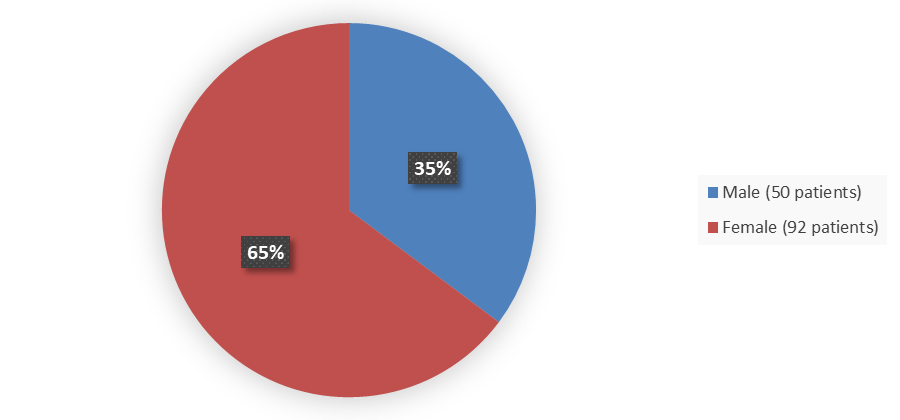
Figure 1 summarizes how many male and female patients were enrolled in the DeFi trial used to evaluate the safety of OGSIVEO.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
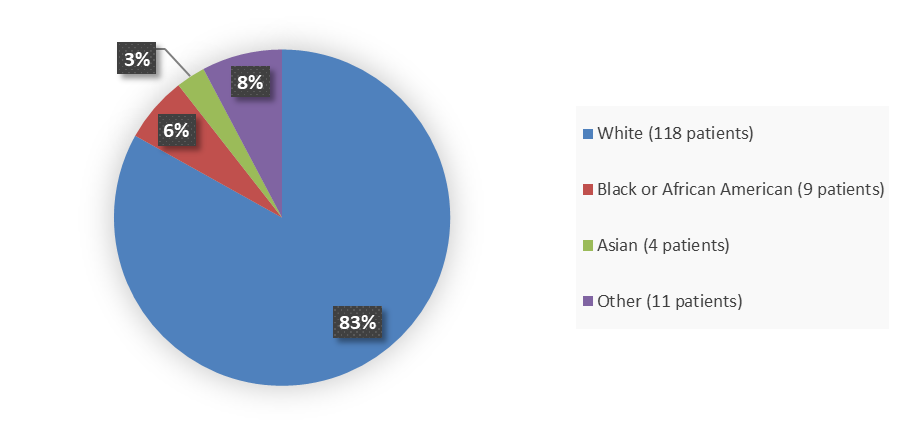
Figure 2 summarizes the percentage of patients by race enrolled in the DeFi trial used to evaluate the efficacy of OGSIVEO.
Figure 2. Baseline Demographics by Race (Efficacy Popualtion)
Source: Adapted from FDA Review
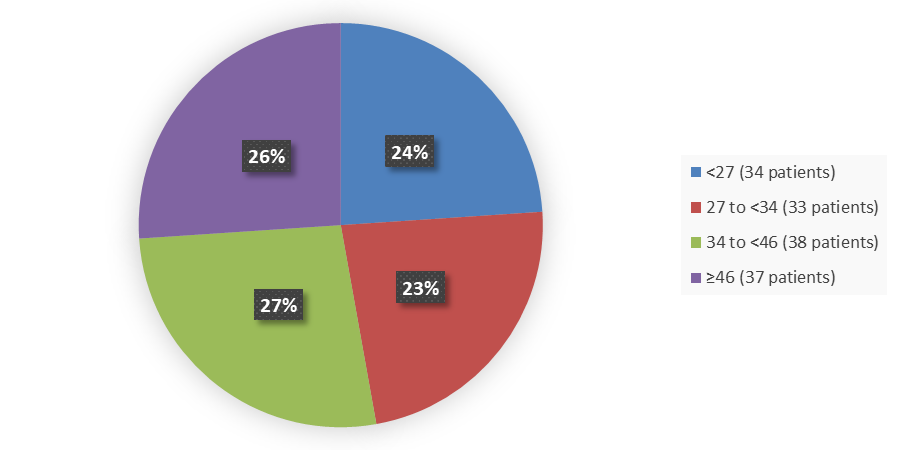
Figure 3 summarizes how many patients by age were in the DeFi trial used to evaluate the side effects of OGSIVEO.
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
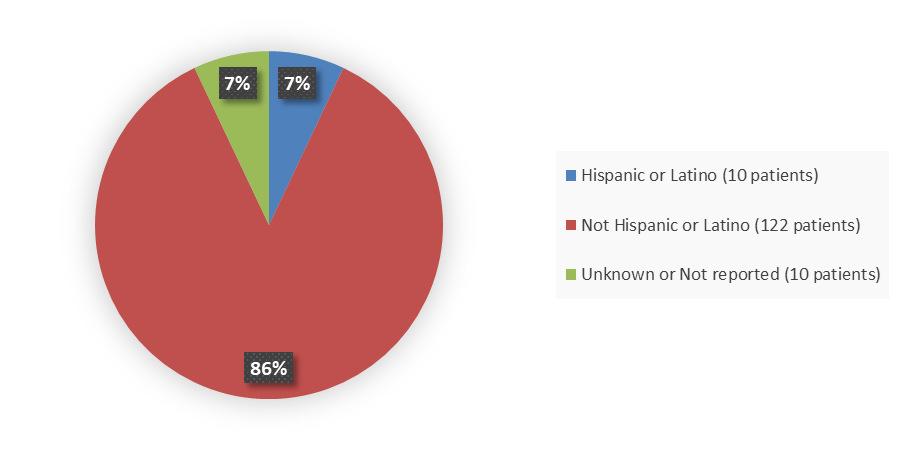
Figure 4 summarizes how many patients by ethnicity were in the DeFi trial used to evaluate the side effects of OGSIVEO.
Figure 4. Baseline Demographics by Ethnicity (Efficacy Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes demographics of all patients in the efficacy population.
Table 1. Demographic Parameters, Efficacy Population
|
Demographic Parameters |
OGSIVEO |
Placebo |
|---|---|---|
|
Sex, n (%) |
||
|
Male |
25 (36) |
25 (35) |
|
Female |
45 (64) |
47 (65) |
|
Age at informed consent, years |
||
|
Median |
33.5 |
34.5 |
|
Min, max |
18, 73 |
18, 76 |
|
Age group, years, n (%) |
||
|
<27 |
20 (29) |
14 (19) |
|
27 to <34 |
15 (21) |
18 (25) |
|
34 to <46 |
13 (19) |
25 (35) |
|
≥46 |
22 (31) |
15 (21) |
|
Race, n (%) |
||
|
White |
64 (91) |
54 (75) |
|
Black or African American |
4 (6) |
5 (7) |
|
Asian |
1 (1.4) |
3 (4.2) |
|
Other |
1 (1.4) |
10 (14) |
|
Ethnicity, n (%) |
||
|
Not Hispanic or Latino |
67 (96) |
55 (76) |
|
Hispanic or Latino |
1 (1.4) |
9 (13) |
|
Unknown |
0 |
3 (4.2) |
|
Not reported |
2 (2.8) |
5 (7) |
Source: Adapted from FDA Review
Table 2 summarizes demographics of all patients in the safety population.
Table 2. Demographic Parameters, Safety Population
|
Demographic Parameters |
OGSIVEO |
Placebo |
|---|---|---|
|
Sex, n (%) |
||
|
Male |
25 (36) |
25 (35) |
|
Female |
44 (64) |
47 (65) |
|
Age at informed consent, years |
||
|
Median |
33.0 |
34.5 |
|
Min, max |
18, 73 |
18, 76 |
|
Age group, years, n (%) |
||
|
<27 |
20 (29) |
14 (19) |
|
27 to <34 |
15 (22) |
18 (25) |
|
34 to <46 |
13 (19) |
25 (35) |
|
≥46 |
21 (30) |
15 (21) |
|
Race, n (%) |
||
|
White |
63 (91) |
54 (75) |
|
Black or African American |
4 (6) |
5 (7) |
|
Asian |
1 (1.5) |
3 (4.2) |
|
Native Hawaiian or other Pacific Islander |
0 |
0 |
|
American Indian or Alaska Native |
0 |
0 |
|
Other |
1 (1.5) |
10 (14) |
|
Ethnicity, n (%) |
||
|
Not Hispanic or Latino |
66 (96) |
55 (76) |
|
Hispanic or Latino |
1 (1) |
9 (13) |
|
Unknown |
0 |
3 (4.2) |
|
Not reported |
2 (2.9) |
5 (7) |
Source: Adapted from FDA Review
What are the benefits of this drug?
In this trial, patients taking OGSIVEO experienced a 71% reduction in the risk of tumor growth, disease worsening, or death after treatment (PFS) compared to patients taking placebo. In addition, 41% of patients taking OGSIVEO experienced complete or partial shrinkage of their tumors compared to 8% of patients taking placebo. These results were supported by an improvement in pain in patients taking OGSIVEO.
What are the benefits of this drug (results of trials used to assess efficacy)?
The results of the clinical trial demonstrated that OGSIVEO provided clinically meaningful and statistically significant improvement in PFS, as well as a statistically significant improvement in tumor shrinkage compared to placebo.
Table 3. Summary of Efficacy Results (Efficacy Population)
|
Efficacy Result |
OGSIVEO |
Placebo |
|---|---|---|
|
Progression-free survival |
||
|
Patients with event, n (%) |
12 (17) |
37 (51) |
|
Radiographic progression1 |
11 (16) |
30 (42) |
|
Clinical progression1 |
1 (1) |
6 (8) |
|
Death |
0 |
1 (1) |
|
Median PFS, months (95% CI)2 |
NR (NR, NR) |
15.1 (8.4, NR) |
|
Hazard ratio (95% CI) |
0.29 (0.15, 0.55) |
|
|
p-value3 |
<0.001 |
|
|
Objective response rate1 |
||
|
Objective response rate, n (%) |
29 (41) |
6 (8) |
|
95% CI4 |
29.8, 53.8 |
3.1, 17.3 |
|
Complete response, n (%) |
5 (7) |
0 |
|
Partial response, n (%) |
24 (34) |
6 (8) |
|
p-value5 |
<0.001 |
|
Source: OGSIVEO Prescribing Information
1 Assessed by blinded independent central review.
2 Obtained using Kaplan-Meier methodology.
3 p-value was from a one-sided stratified log-rank test with placebo as reference.
4 Obtained using exact method based on binomial distribution.
5 p-value was from a two-sided Cochran-Mantel-Haenszel test.
Abbreviations: CI, confidence interval; N, number of patients; NR, not reached; PFS, progression-free survival
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: OGSIVEO worked similarly in males and females.
- Race: The majority of patients in the clinical trial were White. Differences in response to OGSIVEO among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in response to OGSIVEO between patients younger and older than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 4 summarize efficacy results by demographic subgroups. Due to the small sample sizes in some of the subgroups, results should be interpreted with caution.
Table 4. Progression-Free Survival Results by Sex (Efficacy Population)
|
Parameter |
Male |
Female |
||
|---|---|---|---|---|
|
OGSIVEO |
Placebo |
OGSIVEO |
Placebo |
|
|
Median PFS (95% CI) |
NR (22.4, NR) |
NR (5.6, NR) |
NR (NR, NR) |
13.8 (8.3, NR) |
|
Hazard ratio (95% CI) |
0.26 (0.08, 0.82) |
0.30 (0.14, 0.67) |
||
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; N, number of patients; NR, not reached; PFS, progression-free survival
What are the possible side effects?
The most common side effects (≥15%) in patients treated with OGSIVEO are diarrhea, ovarian problems, rash, nausea, tiredness, mouth sores, headache, stomach (abdominal) pain, cough, hair loss, upper respiratory tract infection, and shortness of breath.
OGSIVEO can cause serious side effects including diarrhea, ovarian problems, liver problems, new nonmelanoma skin cancers, and electrolyte (salt) problems.
What are the possible side effects (results of trials used to assess safety)?
The possible side effects occurring in ≥15% of patients treated with OGSIVEO and ≥5% more frequently than in patients treated with placebo are shown in Table 5.
Table 5. Side Effects Observed in ≥15% of Patients Treated With OGSIVEO and ≥5% More Frequently Than in the Placebo Arm
|
|
OGSIVEO, N=69 |
Placebo, N=72 |
||
|---|---|---|---|---|
|
All Grades |
Grade 3 |
All Grades |
Grade 3 |
|
|
Diarrhea |
84 |
16 |
35 |
1.4 |
|
Ovarian toxicity1,2,3 |
75 |
0 |
0 |
0 |
|
Rash1 |
68 |
6 |
14 |
0 |
|
Nausea |
54 |
1.4 |
39 |
0 |
|
Fatigue1 |
51 |
2.9 |
38 |
0 |
|
Stomatitis1 |
39 |
4 |
4 |
0 |
|
Headache1 |
30 |
0 |
15 |
0 |
|
Abdominal pain1 |
22 |
1.4 |
14 |
1.4 |
|
Cough1 |
20 |
0 |
6 |
0 |
|
Alopecia |
19 |
0 |
1.4 |
0 |
|
Upper respiratory tract infection1 |
17 |
0 |
2.8 |
0 |
|
Dyspnea |
16 |
0 |
6 |
0 |
Source: OGSIVEO Prescribing Information
1 Includes multiple related composite terms.
2 Investigator assessment of ovarian toxicity included ovarian failure, premature menopause, amenorrhea, and menopause.
3 The number of females of reproductive potential in each arm is used as the denominator (OGSIVEO N=36, Placebo N=37).
Abbreviations: N, number of patients
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The risk of ovarian problems (ovarian toxicity) applies only to premenopausal females. The risk of other side effects was similar in males and females.
- Race: The majority of patients in the clinical trial were White. Differences in side effects among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects in patients younger and older than 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6 summarize side effects by demographic subgroups. Due to the small sample sizes in some of the subgroups, results should be interpreted with caution.
Table 6. Treatment-Emergent Adverse Events by Sex (Safety Population)
|
Adverse Event |
Male |
Female |
||
|---|---|---|---|---|
|
OGSIVEO |
Placebo |
OGSIVEO |
Placebo |
|
|
Any TEAE |
100 |
96 |
100 |
96 |
|
Patients with TEAEs grade ≥3 |
44 |
24 |
61 |
13 |
|
Patients with serious TEAEs |
16 |
20 |
23 |
6 |
Source: Adapted from FDA Review
Abbreviations: N, number of patients; TEAE, treatment-emergent adverse events
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.