Drug Trials Snapshots: WINREVAIR
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the WINREVAIR Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
WINREVAIR (sotatercept-csrk)
WIN-reh-vair
Merck Sharp & Dohme, LLC
Approval date: March 26, 2024
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
WINREVAIR is indicated for the treatment of adults with pulmonary arterial hypertension (PAH, WHO group 1) to increase exercise capacity, improve function, and reduce clinical worsening.
How is this drug used?
WINREVAIR is administered once every three weeks by subcutaneous injection.
The healthcare provider will decide how many treatment cycles will be given.
Who participated in the clinical trials?
The FDA approved WINREVAIR based on evidence of safety and effectiveness from a clinical trial of 323 patients with PAH (WHO group 1 functional class II or III). The trial was conducted at 126 sites in 21 of countries in Argentina, Australia, Belgium, Brazil, Canada, Czech Republic, France, Germany, Israel, Italy, Mexico, Netherlands, New Zealand, Poland, Serbia, South Korea, Spain, Sweden, Switzerland, United Kingdom, and the United States. The study included 88 patients inside the United States (43 in the WINREVAIR group and 45 in the placebo group).
How were the trials designed?
The study compared WINREVAIR and placebo on 6-minute walk distance (6MWD) at Week 24.
DEMOGRAPHICS SNAPSHOT
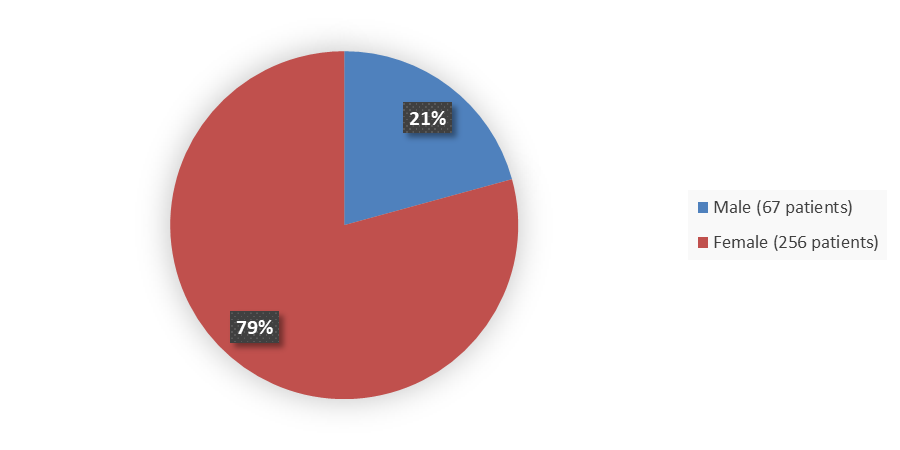
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of WINREVAIR.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
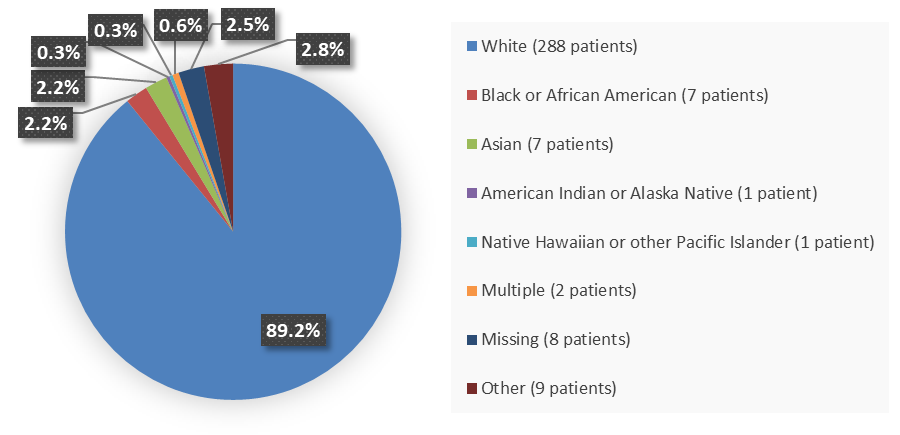
Figure 2 summarizes how many patients by race were enrolled in the clinical trial used to evaluate the efficacy of WINREVAIR.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
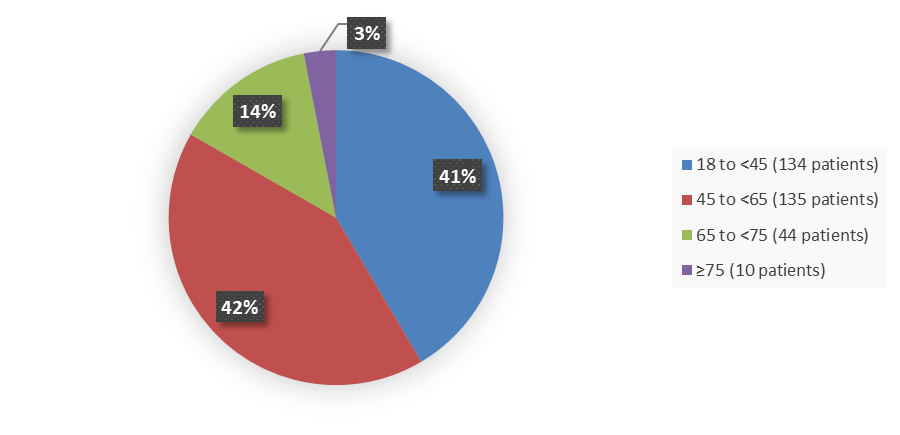
Figure 3 summarizes how many patients by age were enrolled in the clinical trial used to evaluate the efficacy of WINREVAIR.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
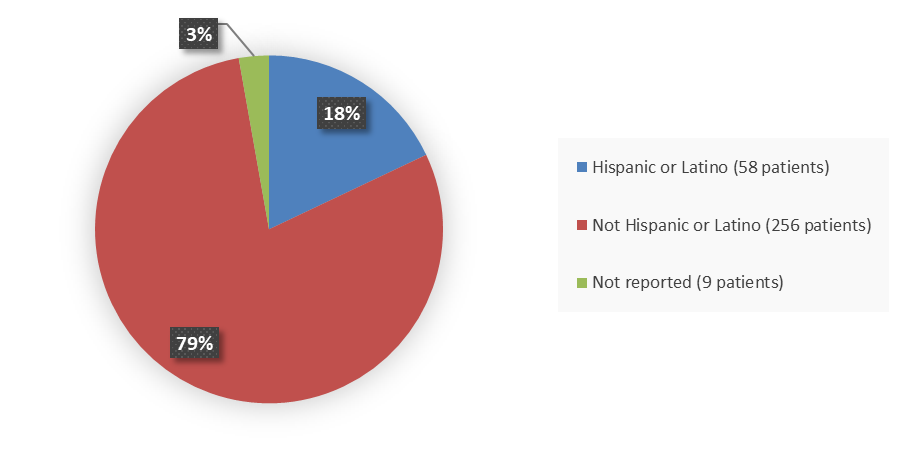
Figure 4 summarizes how many patients by ethnicity were enrolled in the clinical trial used to evaluate the efficacy of WINREVAIR.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics of All Participants
|
Demographic |
WINREVAIR N=163 n (%) |
Placebo N=160 n (%) |
Overall N=323 n (%) |
|
Sex |
|||
|
Female |
129 (79.1) |
127 (79.4) |
256 (79.3) |
|
Male |
34 (20.9) |
33 (20.6) |
67 (20.7) |
|
Race |
|||
|
American Indian or Alaska Native |
0 (0) |
1 (0.6) |
1 (0.3) |
|
Asian |
1 (0.6) |
6 (3.8) |
7 (2.2) |
|
Black or African American |
2 (1.2) |
5 (3.1) |
7 (2.2) |
|
Multiple |
2 (1.2) |
0 (0) |
2 (0.6) |
|
Native Hawaiian or other Pacific Islander |
0 (0) |
1 (0.6) |
1 (0.3) |
|
Other |
5 (3.1) |
4 (2.5) |
9 (2.8) |
|
White |
147 (90.2) |
141 (88.1) |
288 (89.2) |
|
Missing |
6 (3.7) |
2 (1.2) |
8 (2.5) |
|
Age group, years |
|||
|
18 to <45 |
69 (42.3) |
65 (40.6) |
134 (41.5) |
|
45 to <65 |
69 (42.3) |
66 (41.2) |
135 (41.8) |
|
65 to <75 |
20 (12.3) |
24 (15.0) |
44 (13.6) |
|
≥75 |
5 (3.1) |
5 (3.1) |
10 (3.1) |
|
Ethnicity |
|||
|
Hispanic or Latino |
27 (16.6) |
31 (19.4) |
58 (18.0) |
|
Not Hispanic or Latino |
132 (81.0) |
124 (77.5) |
256 (79.3) |
|
Not reported |
4 (2.5) |
5 (3.1) |
9 (2.8) |
|
Country of participation, n (%) |
|
|
|
|
United States |
43 (26.4) |
45 (28.1) |
88 (27.2) |
Source: Adapted from FDA Review
Table 2. Baseline Demographics of Participants Inside the United States
|
Demographic Characteristic |
WINREVAIR N=43 |
Placebo N=45 |
Overall N=88 |
|
Sex, n (%) |
|||
|
Female |
32 (74.4) |
34 (75.6) |
66 (75.0) |
|
Male |
11 (25.6) |
11 (24.4) |
22 (25.0) |
|
Age, years |
|||
|
Mean (SD) |
47.7 (12.2) |
48 (15.2) |
47.9 (13.7) |
|
Median (min, max) |
46 (28, 74) |
48 (22, 77) |
46 (22, 77) |
|
Age group, years, n (%) |
|||
|
18 to <45 |
19 (44.2) |
18 (40.0) |
37 (42.0) |
|
45 to <65 |
18 (41.9) |
18 (40.0) |
36 (40.9) |
|
65 to <75 |
6 (14.0) |
8 (17.8) |
14 (15.9) |
|
≥75 |
0 (0) |
1 (2.2) |
1 (1.1) |
|
Race, n (%) |
|||
|
Asian |
1 (2.3) |
2 (4.4) |
3 (3.4) |
|
Black or African American |
2 (4.7) |
2 (4.4) |
4 (4.5) |
|
Multiple |
2 (4.7) |
0 (0) |
2 (2.3) |
|
Native Hawaiian or other Pacific Islander |
0 (0) |
1 (2.2) |
1 (1.1) |
|
Other |
2 (4.7) |
1 (2.2) |
3 (3.4) |
|
White |
36 (83.7) |
39 (86.7) |
75 (85.2) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
10 (23.3) |
8 (17.8) |
18 (20.5) |
|
Not Hispanic or Latino |
33 (76.7) |
37 (82.2) |
70 (79.5) |
|
Country of participation, n (%) |
|||
|
United States |
43 (100) |
45 (100) |
88 (100) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
After 24 weeks, patients assigned to WINREVAIR could walk further than patients assigned to placebo, physicians more often rated patients on WINREVAIR as clinically improved, and the risk of death or clinical worsening was reduced.
What are the benefits of this drug (results of trials used to assess efficacy)?
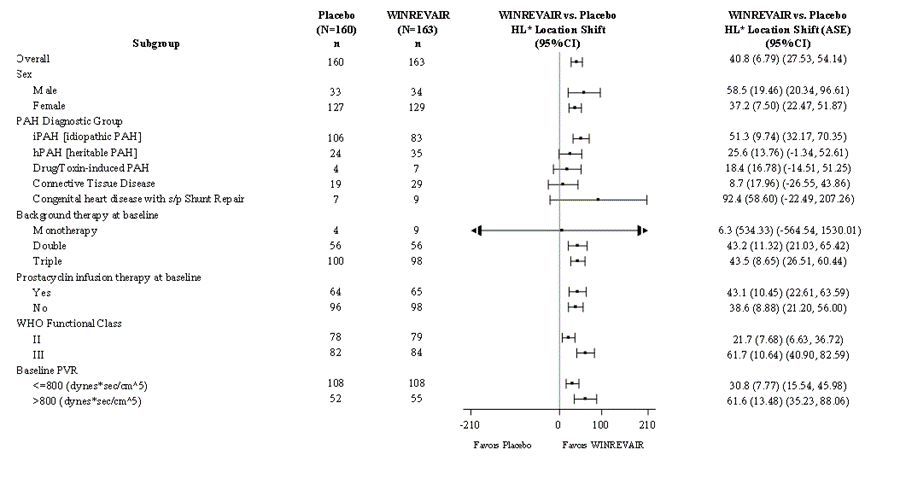
The primary efficacy endpoint was the change from baseline at Week 24 in 6-Minute Walk Distance (6MWD). In the WINREVAIR treatment group, the placebo-adjusted median increase in 6MWD was 41 meters (95% CI: 28, 54; p<0.001). Figure 5 displays placebo-adjusted changes in 6MWD at Week 24 in relevant subgroups.
Treatment with WINREVAIR resulted in an 84% reduction in the occurrence of death from any cause or PAH clinical worsening events compared to placebo (Table 3). Clinical worsening events included worsening-related listing for lung and/or heart transplant, need to initiate rescue therapy with an approved background PAH therapy or the need to increase the dose of infusion prostacyclin by ≥10%, need for atrial septostomy, hospitalization for worsening PAH (≥24 hours), or deterioration of PAH (worsened WHO functional class and decrease in 6MWD ≥15% with both events occurring at the same time or different times). Clinical worsening events and death were captured until the last patient completed the Week 24 visit (data up to the data cutoff; median duration of exposure 33.6 weeks).
Figure 5. Change From Baseline in 6-Minute Walk Distance (Meters) at Week 24 in Subgroups
Source: WINREVAIR Prescribing Information
* Hodges-Lehmann (HL) location shift from placebo estimate (median of all paired differences).
Abbreviations: ASE, asymptotic standard error; CI, confidence interval; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; s/p, status post
Table 3. Death From Any Cause or PAH Clinical Worsening Events
|
Cause of Death |
Placebo N=160 |
WINREVAIR N=163 |
|
Number of subjects who experienced death or at least one clinical worsening event, n (%) |
42 (26.3) |
9 (5.5) |
|
Hazard ratio (95% CI) |
0.16 (0.08, 0.35) |
|
|
p-value |
p<0.001 |
|
|
Assessment of clinical worsening events1, n (%) |
||
|
Death |
7 (4.4) |
2 (1.2) |
|
Worsening-related listing for lung and/or heart transplant |
2 (1.3) |
1 (0.6) |
|
Need to initiate rescue therapy with an approved PAH therapy or the need to increase the dose of infusion prostacyclin by 10% or more |
17 (10.6) |
2 (1.2) |
|
Need for atrial septostomy2 |
0 (0.0) |
0 (0.0) |
|
PAH-specific hospitalization (≥24 hours) |
8 (5.0) |
0 (0.0) |
|
Deterioration of PAH3 |
15 (9.4) |
4 (2.5) |
Source: Adapted from WINREVAIR Prescribing Information
1 A subject can have more than one assessment recorded for their clinical worsening.
2 There were no events of atrial septostomy.
3 Deterioration of PAH therapy is defined by both of the following events occurring at any time, even if they began at different times, as compared to their baseline values: (a) Worsened WHO functional class (II to III, III to IV, II to IV, etc.); and (b) Decrease in 6MWD by ≥15% (confirmed by two 6MWTs at least 4 hours apart but no more than one week).
Abbreviations: 6MWT, 6-minute walk test; CI, confidence interval; PAH, pulmonary arterial hypertension
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: WINREVAIR worked similarly in males and females. The majority of participants in the clinical trial were female.
- Race: The number of patients of races other than White was small; therefore, differences in how WINREVAIR worked among races could not be determined.
- Ethnicity: WINREVAIR worked similarly in Hispanic or Latino and Not Hispanic or Latino patients.
- Age: The effect of WINREVAIR was similar in patients at 18 to <45 years of age, 45 to <65 years of age, 65 to <75 years of age, and older than 75 years of age.
- U.S. versus Non-U.S.: WINREVAIR worked similarly in populations in and outside the United States.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 4. Change From Baseline in 6-Minute Walk Distance (Meters) at Week 24 in Subgroups
|
Subgroup |
Median Distance (Meters) (95% CI) |
|
Sex |
|
|
Female |
39 (24, 53) |
|
Male |
49 (21, 85) |
|
Age group, years |
|
|
18 to <45 |
49 (28, 69) |
|
45 to <65 |
37 (21, 53) |
|
65 to <75 |
23 (-22, 58) |
|
≥75 |
36 (-52, 122) |
|
Ethnicity |
|
|
Hispanic or Latino |
53 (22, 94) |
|
Not Hispanic or Latino |
37 (23, 51) |
|
Region |
|
|
United States |
42 (17, 67) |
|
Non-United States |
37 (21, 53) |
Source: Adapted from FDA Review
What are the possible side effects?
The most common side effects were headache, nose bleeding, diarrhea, dizziness, telangiectasia (spider veins), rash, and erythema.
WINREVAIR causes increases in hemoglobin (red blood cells). High concentrations of red blood cells in blood may increase the risk of blood clots.
WINREVAIR causes decreases in platelet count, which can result in bleeding problems.
Based on findings in animal studies, WINREVAIR may impair female and male fertility and cause fetal harm when administered during pregnancy.
What are the possible side effects (results of trials used to assess safety)?
Table 5 shows the most common (≥10% in patients receiving WINREVAIR and 5% more than placebo) adverse reactions in the clinical trial.
In the clinical trial, moderate elevations in hemoglobin (>2 g/dL above upper limit of normal [ULN]) occurred in 15% of patients taking WINREVAIR while no elevations ≥4 g/dL above ULN were observed.
In the clinical trial, severe reduction in platelet count occurred in 3% of patients taking WINREVAIR. Thrombocytopenia occurred more frequently in patients also receiving prostacyclin infusion.
In the clinical trial, serious bleeding events (e.g., gastrointestinal, intracranial hemorrhage) were reported in 4% of patients taking WINREVAIR and 1% of patients taking placebo. Patients with serious bleeding events were more likely to be on prostacyclin background therapy and/or antithrombotic agents, or have low platelet counts.
Table 5. Adverse Reactions ≥10% in Patients Receiving WINREVAIR and at Least 5% More Than Placebo
|
Adverse Reaction |
Placebo N=160 n (%) |
WINREVAIR N=163 n (%) |
|
Headache |
28 (17.5) |
41 (25.2) |
|
Epistaxis |
3 (1.9) |
36 (22.1) |
|
Rash |
13 (8.1) |
33 (20.2) |
|
Telangiectasia |
7 (4.4) |
27 (16.6) |
|
Diarrhea |
16 (10.0) |
25 (15.3) |
|
Dizziness |
10 (6.2) |
24 (14.7) |
|
Erythema |
5 (3.1) |
22 (13.5) |
Source: WINREVAIR Prescribing Information
Were there any differences in side effects among sex, race, and age?
- Sex: The number of male patients was small; therefore, differences in side effects between sexes could not be determined.
- Race: The number of patients of races other than White was small; therefore, differences in side effects among races could not be determined.
- Age: The number of patients of age above 65 years was small; therefore, differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6 and Table 7 show summary of adverse events by demographic for all grade adverse events and grade 3 and grade 4 adverse events. The majority of patients were female (79%), White (89%), and younger than 65 years of age (83%).
Table 6. All Grade Adverse Events by Sex, Race, Age, and Ethnicity
|
Demographic |
WINREVAIR N=163 n/Ns (%) |
Placebo N=160 n/Ns (%) |
Overall N=323 n/Ns (%) |
|
Sex |
|||
|
Female |
123/129 (95.3) |
119/127 (93.7) |
242/256 (94.5) |
|
Male |
28/34 (82.4) |
30/33 (90.9) |
58/67 (86.6) |
|
Race |
|||
|
American Indian or Alaska Native |
0/0 (NA) |
1/1 (100) |
1/1 (100) |
|
Asian |
1/1 (100) |
6/6 (100) |
7/7 (100) |
|
Black or African American |
2/2 (100) |
5/5 (100) |
7/7 (100) |
|
Multiple |
1/2 (50.0) |
0/0 (NA) |
1/2 (50.0) |
|
Native Hawaiian or other Pacific Islander |
0/0 (NA) |
1/1 (100) |
1/1 (100) |
|
Other |
5/5 (100) |
4/4 (100) |
9/9 (100) |
|
White |
136/147 (92.5) |
130/141 (92.2) |
266/288 (92.4) |
|
Missing |
6/6 (100) |
2/2 (100) |
8/8 (100) |
|
Age group, years |
|||
|
18 to <45 |
64/69 (92.8) |
61/65 (93.8) |
125/134 (93.3) |
|
45 to <65 |
65/69 (94.2) |
61/66 (92.4) |
126/135 (93.3) |
|
65 to <75 |
19/20 (95.0) |
22/24 (91.7) |
41/44 (93.2) |
|
≥75 |
3/5 (60.0) |
5/5 (100) |
8/10 (80.0) |
|
Ethnicity |
|||
|
Hispanic or Latino |
26/27 (96.3) |
31/31 (100) |
57/58 (98.3) |
|
Not Hispanic or Latino |
121/132 (91.7) |
114/124 (91.9) |
235/256 (91.8) |
|
Not reported |
4/4 (100) |
4/5 (80.0) |
8/9 (88.9) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients meeting criteria; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
Table 7. Grade 3 or 4 Adverse Events by Sex, Race, Age, and Ethnicity
|
Demographic |
WINREVAIR N=163 n/Ns (%) |
Placebo N=160 n/Ns (%) |
Overall N=323 n/Ns (%) |
|
Sex |
|||
|
Female |
18/129 (14.0) |
24/127 (18.9) |
42/256 (16.4) |
|
Male |
4/34 (11.8) |
6/33 (18.2) |
10/67 (14.9) |
|
Race |
|||
|
American Indian or Alaska Native |
0/0 (NA) |
0/1 (0) |
0/1 (0) |
|
Asian |
0/1 (0) |
0/6 (0) |
0/7 (0) |
|
Black or African American |
0/2 (0) |
2/5 (40.0) |
2/7 (28.6) |
|
Multiple |
0/2 (0) |
0/0 (NA) |
0/2 (0) |
|
Native Hawaiian or other Pacific Islander |
0/0 (NA) |
0/1 (0) |
0/1 (0) |
|
Other |
3/5 (60.0) |
1/4 (25.0) |
4/9 (44.4) |
|
White |
19/147 (12.9) |
26/141 (18.4) |
45/288 (15.6) |
|
Missing |
0/6 (0) |
1/2 (50.0) |
1/8 (12.5) |
|
Age group, years |
|||
|
18 to <45 |
9/69 (13.0) |
9/65 (13.8) |
18/134 (13.4) |
|
45 to <65 |
6/69 (8.7) |
11/66 (16.7) |
17/135 (12.6) |
|
65 to <75 |
7/20 (35.0) |
7/24 (29.2) |
14/44 (31.8) |
|
≥75 |
0/5 (0) |
3/5 (60.0) |
3/10 (30.0) |
|
Ethnicity |
|||
|
Hispanic or Latino |
3/27 (11.1) |
3/31 (9.7) |
6/58 (10.3) |
|
Not Hispanic or Latino |
19/132 (14.4) |
24/124 (19.4) |
43/256 (16.8) |
|
Not reported |
0/4 (0) |
3/5 (60.0) |
3/9 (33.3) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients meeting criteria; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION