Submit Documents via the CTP Portal
On this page:
- What Is CTP Portal?
- What Can I Do in the CTP Portal?
- Managing CTP Portal User Accounts
- How Do I Create a New User Account in the CTP Portal?
- How Do I Obtain a CTP Portal Account or Submit Help Desk Inquiries?
- What Responsibilities Does My Organization Have in Managing User Accounts for CTP Portal?
- When Setting Privileges in the CTP Portal, How Do I Know Which Specific Privileges Each User Has?
- Does CTP Portal Have Non-IAM Privileges?
- FDA's eSubmitter Software and the CTP Portal
What Is CTP Portal?
CTP Portal is a web-based tool which enables regulated tobacco industry, including manufacturers, importers, and distributors, to electronically send tobacco product submissions (packaged by eSubmitter) to FDA. The CTP Portal can also be used to facilitate secure two-way messaging, allowing tobacco industry users to access information regarding their submissions and contact their FDA representative. Designating an Industry Account Manager (IAM) for your organization is the only way to create and access a CTP Portal account for your organization.
CTP Portal
What Can I Do in the CTP Portal?
The CTP Portal is intended to be used by regulated tobacco industry manufacturers, importers, distributors, TPMF owners, universities, and other sponsors who send tobacco product submissions to CTP.
The CTP Portal can be used to:
- Upload documents to CTP electronically and securely, seven days a week, with confirmation of receipt, if uploaded successfully, including:
- Ingredient Listing
- Reporting of Harmful and Potentially Harmful Constituents (HPHCs)
- Health Documents (not adverse experience or product problem reports)*
- Apply to Market
- Respond to CTP inquiries
- View a list of your submissions and FDA regulatory letters, including:
- Date CTP received your submission
- Assigned FDA tracking number for your submission
* For information regarding section 904(a)(4) requirements, please refer to the Final Guidance: Health Document Submission Requirements for Tobacco Products.
Watch video: Tips for Applicants: Using the CTP Portal, which provides an overview of the CTP Portal and how to use it, including how to find your application’s submission tracking number (STN) online.
The CTP Portal cannot be used for the following:
- Register tobacco product manufacturing establishment(s) and list your product(s)
- The CTP Portal does not replace the Tobacco Registration and Product Listing Next Gen (TRLM NG) system. Refer to the TRLM NG Instructions for guidance on how to submit tobacco registration and product listing information. Note: Users must first create a TRLM NG account before submitting tobacco registration and product listings.
- Gain access to the FDA Electronic Submissions Gateway (ESG)
- Gain access to the Safety Reporting Portal (SRP) for submitting Adverse Experience (AE) and Product Problem Reports
Managing CTP Portal User Accounts
The organization is responsible for designating the Industry Account Manager (IAM), and the designated IAM is responsible for managing the CTP Portal user accounts. While the IAM is the delegate authority, ultimately, the organization is responsible for the behavior of their employees and agents on the CTP Portal.
How Do I Create a New User Account in the CTP Portal?
Only the organization’s IAM(s) can create a new user account.
How Do I Obtain a CTP Portal Account or Submit Help Desk Inquiries?
Before requesting a CTP Portal account, inquire within your organization whether an IAM has been designated. If your organization already has an IAM, contact them to request a user account. Otherwise, request an Industry Account Manager (IAM) for your organization.
For additional inquiries about CTP Portal accounts, contact the FDA eSub Help Desk by email at CTPeSub@fda.hhs.gov and include "CTP Portal" in the subject line or call 1-877-CTP-1373, extension 4.
When contacting the FDA eSub Help Desk to submit a ticket, please include the following information if applicable:
- Name
- Company
- Phone number
- Username
- STN (tracking number)
- A summary of the issue including what IT application is being used, URL of the site where the issue was encountered, the web browser being used, and whether the computer is a Windows or Mac.
- For Industry Account Manager (IAM) applicants, please include a tracking number and date of application.
What Responsibilities Does My Organization Have in Managing User Accounts for CTP Portal?
Your organization is responsible for creating users and managing the list of users who have access to your organization’s CTP Portal account and managing the permissions of each user. If an employee, attorney, or agent are no longer affiliated with your organization, the IAMs for your organization are responsible for deactivating that person’s CTP Portal user account to prevent further access. Alternatively, if the sole IAM is departing your organization, it is the responsibility of your organization and the departing IAM to reassign the IAM role to another individual prior to their departure. This will ensure continuity of access and permissions for your organization.
When Setting Privileges in the CTP Portal, How Do I Know Which Specific Privileges Each User Has?
CTP Portal has five different privileges that can be given to each CTP Portal user by the CTP Portal Admin. The different privileges and their permissions and abilities within CTP Portal are described in the table below:
| CTP Portal "Privilege" | Associated Permission or Ability in CTP Portal |
|---|---|
|
CTP Portal View Submission-Related Information |
Ability to view all of their organization’s non-message-related data and view details of uploaded submissions in CTP Portal. |
|
CTP Portal View/Reply Messages |
Ability to view all of their organization’s message-related data in CTP Portal and reply to messages from CTP related to uploaded submissions. |
|
CTP Portal View Only Messages |
Ability to view their organization’s message-related data in CTP Portal, but no ability to reply to these messages. |
|
CTP Portal Upload |
Ability to submit data to CTP via the CTP Portal Upload functionality. |
|
CTP Portal User Admin |
This is the “privilege” that the IAM needs to be granted in CTP Portal in order to be able to create, manage, and provide general CTP Portal account management support for their organization. |
Does CTP Portal Have Non-IAM Privileges?
Yes, a user can be granted any of the following privileges in CTP Portal:
- View Submission-Related Information: this default privilege allows users to view submission-related information.
- View Only Messages: allows users to view messages sent between FDA and your organization.
- View/Reply Messages: allows users to view and reply to messages sent by FDA.
- Upload: allows users to upload eSubmitter files through CTP Portal.
- User Admin: allows users to create and manage user accounts.
FDA's eSubmitter Software and the CTP Portal
How Do I Package a Submission Using eSubmitter?
You need to use FDA's eSubmitter software:
- Save your text documents as .pdf with optical character recognition and your data files in a data file format such as .xlsx or .xpt. FDA forms in spreadsheet format should be saved as .xlsx. Save all of your submission files to one folder location on your computer.
- Download and install eSubmitter, if you have not already done so.
Note: eSubmitter is a free tool that helps you create an electronic submission, which you can upload into the CTP Portal. - Open eSubmitter.
- Click "create new submission." Select the eSubmitter template for the submission type you want to create:
- CTP Tobacco Product Ingredient Listing
- CTP Reporting of Harmful and Potentially Harmful Constituents (HPHCs)
- CTP Tobacco Product Health Documents
- CTP Transmittal Form for All Other Submission Types
- Follow the guided process within eSubmitter. Answer the questions and fill out the screens. You will be prompted to attach the PDF documents and data files you saved in step 1.
- At the end of the process, eSubmitter packages your submission as a compressed ZIP file. Save the eSubmitter ZIP package to your computer.
How Do I Upload eSubmitter Packages to the CTP Portal?
- Login to CTP Portal.
- Click Launch Upload Tool The system will launch the Upload Tool in a new browser window.
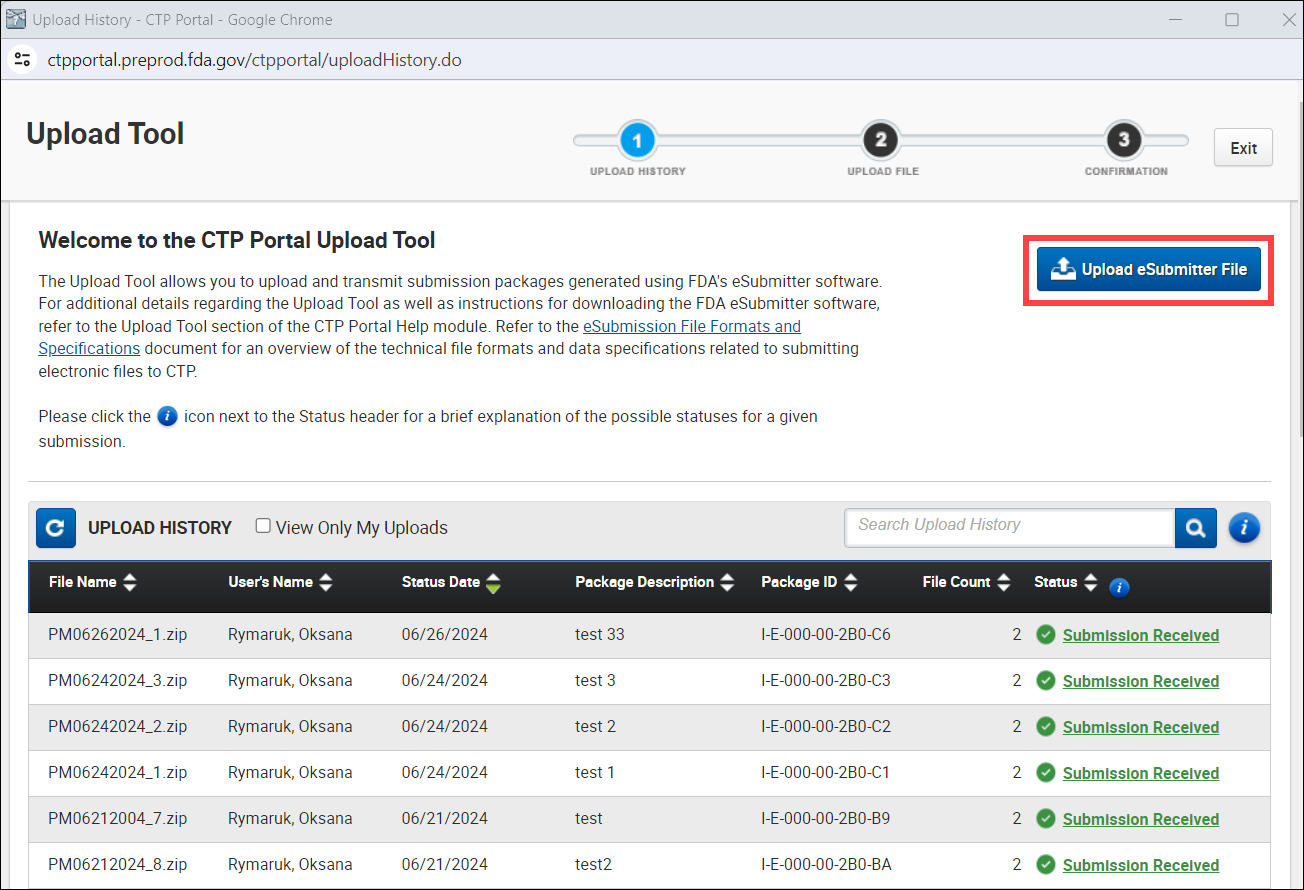
- Click the Upload eSubmitter File button to upload your packages (see image below).
- Browse your computer to locate your eSubmitter file and upload it to the site.
Note: A message will appear in the CTP Portal confirming successful upload. If you do not receive confirmation of your upload or you receive an error message, FDA has not received your package and you will need to resubmit.
* The CTP Portal provides more functionality than the ESG, and CTP recommends using the CTP Portal. However, if you already have an ESG WebTrader account, you can still use it to submit documents to CTP.
Where Can I View Information on Prior Uploads?
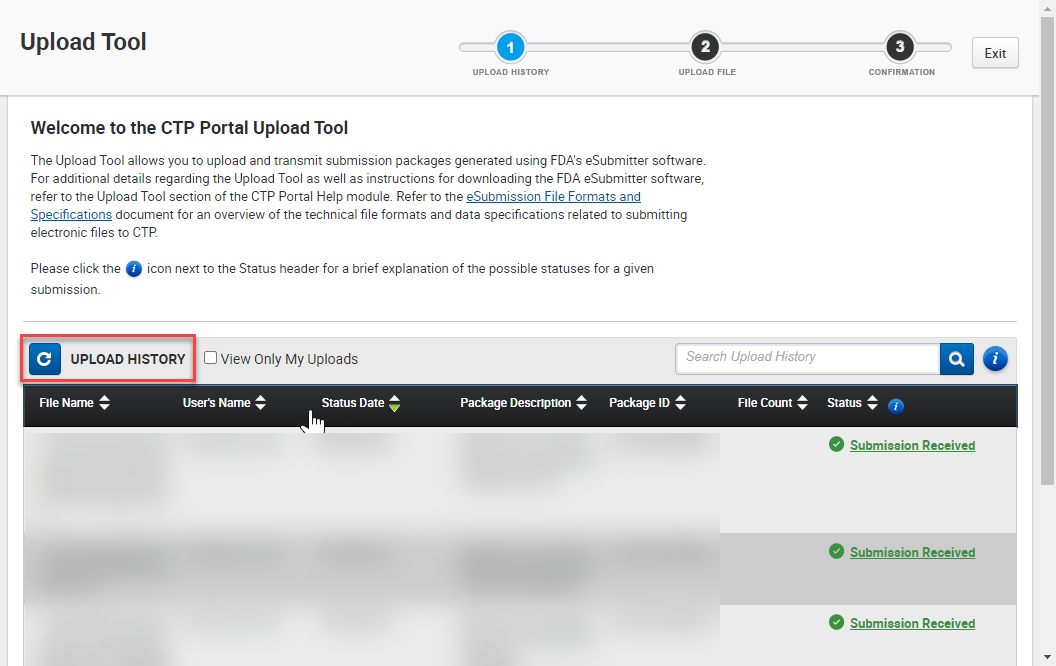
You can view prior uploads within the Upload Tool, under Upload History (see image below).
What Are Some Possible Causes of Being Unable to View My Organization's Uploads?
There are several possible reasons for being unable to view your organization’s uploads, including:
- The name of the organization used to set up the IAM account must be an EXACT match to the organization name submitted in the application.
- There were multiple IAM accounts created for your organization by mistake (e.g., accounts created using slightly different spelling). In this case, please contact the CTP eSub Help Desk by email at CTPeSub@fda.hhs.gov (include "CTP Portal" in the subject line) or call 1-877-CTP-1373, extension 4.
- Your access was removed by the IAM and you no longer have access to view these uploads.
- Your account was deactivated. User account passwords need to be reset every 90 days to stay active (per FDA security protocols). If you did not reset your password in this timeframe, please contact the CTP eSub Help Desk by email at CTPeSub@fda.hhs.gov (include "CTP Portal" in the subject line) or call 1-877-CTP-1373, extension 4.
- Your account was administratively closed by an Industry Account Manager (IAM). In this case, please contact the IAM Request Team by email at ctp-iam-request@fda.hhs.gov for further information.