FDA's Remote Oversight Tools
At the onset of the COVID-19 pandemic, the FDA expanded its use of a variety of surveillance tools and developed new oversight approaches to enable the agency to provide oversight to as many facilities as possible, while utilizing our resources to protect and promote public health.
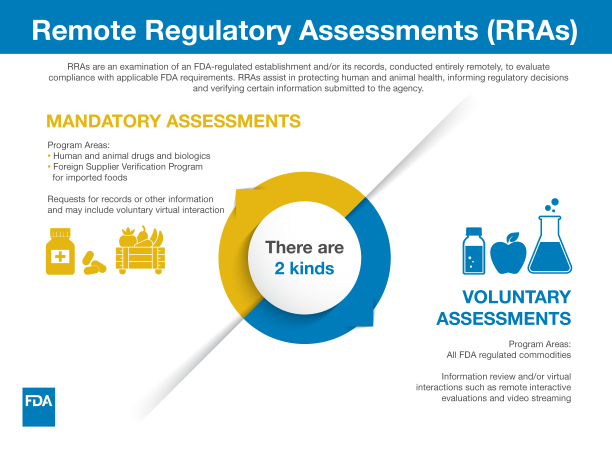
Remote regulatory assessments (RRAs) include voluntary interactive evaluations (such as remote livestreaming video of operations, teleconferences and screen sharing) in addition to requests to review records and other information under existing statutory or regulatory authority. Throughout the pandemic, the FDA has used these tools, domestically and abroad, to help the agency conduct oversight, mitigate risk, and meet critical public health needs.
The agency uses RRAs when we determine that an RRA is an appropriate step in assessing compliance with FDA regulations. Through RRAs, we can (among other things):
- assess compliance of FDA-regulated products and manufacturing processes, which may enable a firm to make corrective actions prior to their next inspection;
- assess the adequacy of corrective actions taken in response to previous inspections of compliant manufacturers, which may increase the time before FDA inspects a facility again or shorten the time of the next inspection;
- if appropriate, make decisions related to applications submitted to the agency without conducting an inspection; and
- identify unreported adverse events or incomplete corrective actions, which resulted in the agency pivoting its oversight approach to conduct an inspection and take a regulatory action.
The agency released a draft guidance – Conducting Remote Regulatory Assessments Questions and Answers – on the expanded use of RRAs and how the FDA generally intends this tool, once finalized, to be incorporated consistently across all FDA-regulated products beyond the current COVID-19 public health emergency.
The agency uses robust oversight tools to protect public health.