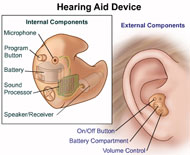
Hearing Aids
UPDATE - August 16, 2022: The FDA issued a final rule: Establishing Over-the-Counter Hearing Aids to improve access to safe, effective, and affordable hearing aids for millions of Americans. This action enables consumers 18 years of age and older with perceived mild to moderate hearing loss to purchase hearing aids directly from stores or online retailers without the need for a medical exam, prescription, or a fitting adjustment by an audiologist.
Concurrently with issuing the final rule, the FDA also issued the final guidance: Regulatory Requirements for Hearing Aid Devices and Personal Sound Amplification Products to clarify the differences between hearing aids, which are medical devices, and personal sound amplification products, which are not regulated as medical devices, and which help people with normal hearing amplify sounds in certain environments.
Read more in the FDA's press release: FDA Finalizes Historic Rule Enabling Access to Over-the-Counter Hearing Aids for Millions of Americans.
Having trouble hearing? Close to 30 million adults in the United States have some degree of hearing loss. Hearing loss can have a negative effect on communication, relationships, school or work performance, and emotional well-being. However, hearing loss doesn't have to restrict your daily activities. Properly fitted hearing aids and aural rehabilitation can help in many listening situations. Aural rehabilitation is the use of techniques to identify and diagnose hearing loss and implement therapies for patients who are hard of hearing, which often involves the use of hearing aids. Aural rehabilitation helps a person focus on adjusting to their hearing loss and the use of their hearing aids. It also explores assistive devices to help improve communication. Most people who are hearing-impaired will need two hearing aids because both ears are often affected by hearing loss, though some people may only need one hearing aid.
This site includes information on the difference between hearing aids, which are intended for use by people with hearing loss, and personal sound amplification products (PSAP), which are intended for consumers with no hearing loss who want to make sounds louder in certain environments, such as for recreational activities like birdwatching or hunting. While the FDA regulates hearing aids as medical devices, PSAPs are not medical devices.
This site provides general information on hearing aids and their benefits, types of hearing loss, procedures to improve hearing, and a checklist of steps to remember and consider before purchasing hearing aids. This site also provides some information about other hearing devices and products, such as PSAPs, that are not intended to improve hearing loss. This site is not intended to provide medical advice. If you have questions about your health, the best source of information is your hearing health care professional.
| Over-the-Counter (OTC) Hearing Aids |
Prescription Hearing Aids (Any hearing aids that do not meet OTC requirements) |
Personal Sound Amplification Products |
|
|---|---|---|---|
| Type of Product | Medical device Electronic product |
Medical device Electronic product |
Electronic product |
| Intended Users |
|
|
People of any age with normal hearing to amplify sounds in certain environments |
| Conditions for Sale |
|
|
No applicable FDA requirements regarding conditions for sale |
Related Information
- Establishing Over-the-Counter Hearing Aids - Final Rule
- Regulatory Requirements for Hearing Aid Devices and Personal Sound Amplification Products - Final Guidance for Industry and Food and Drug Administration Staff
- Hearing Aids and Personal Sound Amplification Products: What to Know