Through-Container Quantitative Screening of Hand Sanitizers
CDER researchers have used a recent innovation in Raman spectroscopy to improve our ability to screen hand sanitizers.
Hand Hygiene and COVID-19
Good hand hygiene has been an important part of the response to the COVID-19 pandemic.1 Washing hands with soap and water for 20 seconds is highly recommended to help prevent spreading disease, especially before eating and after coughing, sneezing, blowing one’s nose or going to the bathroom. If soap and water are not readily available, use of alcohol (ethanol or isopropanol) based hand sanitizers has been recommended by the World Health Organization (WHO)2 and the Center for Disease Control (CDC)3 at concentrations of 60-80 % by volume. Due to the public health emergency posed by COVID-19, consumers and health care providers had difficulty accessing alcohol-based hand sanitizer products early in the pandemic, prompting increases in both the domestic production of hand sanitizers and the importation of sanitizer products from abroad. Some of these hand sanitizer products (domestic or imported) were later found to be contaminated (with methanol or 1-propanol) or otherwise out of specification.4
Even when used as directed, hand sanitizer products contaminated with methanol can cause chronic toxicity, and the ingestion of these contaminated products can be fatal.5 Moreover, alcohol-based hand sanitizers are recommended for use at certain concentrations of ethanol or isopropanol, and products that are out of specification (e.g., sub-potent) may pose risks. Unfortunately, adequate testing of these products, using standard gas chromatography, is time-consuming and often laborious. Thus, with the onset of the COVID-19 pandemic, a rapid screening method to identify low-quality products readily for subsequent analysis and market removal was urgently needed.
Rapid Screening of Hand Sanitizer Products
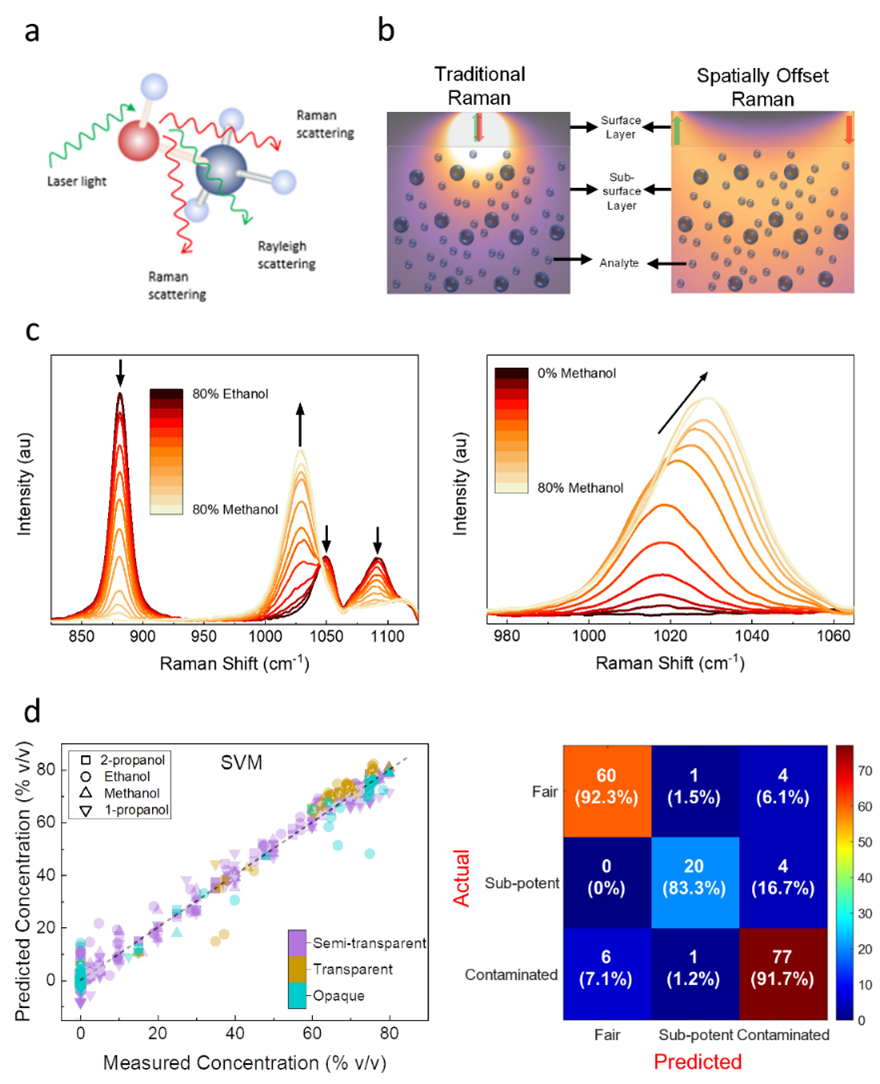
Rapid screening tools such as portable X-ray fluorometers and infrared and Raman spectrometers are commonly employed at ports, borders, and security checkpoints. In rapid screening, much of the focus recently has been on through-container spectroscopy, because such measurements require no sample preparation and allow analysis without opening and potentially spoiling products. Spatially offset Raman spectroscopy (SORS; see Figure 1), however, is a technique that allows acquisition of a molecular fingerprint spectrum of a product through a container (without the need of opening the container to remove a sample of the product). Laser radiation from SORS devices can penetrate most plastic and glass containers (except those expressly designed to block infrared and visible light). In this way, chemical characterization of the product is made by comparing, through use of computer algorithms, the SORS-determined spectrum of the tested product against a trusted library of spectra and thereby chemically identify the product within its container. Depending on formulation, alcohol-based hand sanitizers may contain multiple ingredients in small amounts, such as hydrogen peroxide, glycerin, aloe, polymers, and oils. SORS-determined spectral peaks correspond to the varying ingredients in the product.
Quantitative Through-Container Analysis
Commercially available products such as hand sanitizers can be found in various sizes, shapes, and types of containers. Because different containers allow transmission of light in different amounts, the exact intensity of light interacting with the contents in through-container analysis will not be known, and hence quantification of the contents is not directly possible. However, matrix effects in finished products, such as spectral shifts in alcohol-water mixtures (i.e., where peaks in the observed spectra) can be utilized with advanced chemometrics to allow relative quantitative analysis, independent of the amount of light transmitted through a container.
CDER researchers used SORS and support vector machine (SVM)-based regression algorithms to quantify the amounts of alcohol in hand sanitizer formulations in containers of differing material, thickness, or opacity. The researchers’ analyses took advantage of the concentration dependence of the spectral shifts in alcohol-water mixtures as well as relative intensities of alcohols present. Focusing on the common adulterants methanol and 1-propanol, as well as the acceptable ingredients ethanol and isopropanol, CDER researchers quantified the contents of commercial (gel or liquid) and in-house-made hand sanitizer samples with high accuracy (< 5% root mean-square error). Among 53 different commercial products, only two did not yield a high-quality spectrum using SORS.
In the CDER study,6 active ingredients (e.g., type of alcohol) of 173 commercial and in-house products were identified and quantified regardless of the container material or opacity. The method further allowed for the correct identification of 77 of 84 (92%) samples known to be contaminated with methanol or 1-propanol.
Screening medical countermeasures and other products rapidly and non-invasively can be critical to overcoming challenges in drug shortages and quality of drug products during public health emergencies. Using the method developed in this study, non-invasive, through-container quantitative analysis can be completed within seconds and help sort samples for confirmatory testing by GC or other techniques.
How can this research advance public health?
Screening products rapidly and non-invasively can be critical to overcoming challenges in drug shortages and product quality, particularly in instances where an extremely large number of medical countermeasures such as drug products, sanitation supplies, or vaccines may need to be rapidly tested. Using the method developed in this study and applied to quality investigations of hand sanitizers, non-invasive, through-container quantitative analysis can be readily completed, without opening product containers to extract product sample.
Figure 1. CDER’s Use of Raman Spectroscopy in Hand Sanitizer Investigation. a) In Raman spectroscopy, light that interacts with the chemical bonds of a molecule produces scattered light of frequencies that depend on the nature of the chemical bond. b) In traditional Raman, the scattered light is measured at the point at which the laser light enters the sample. In spatially offset Raman, the scattered light is collected at a lateral offset where photons that travel longer can be detected, and this allows one to obtain information about the molecules at distance from the surface of the container. c) The Raman spectra of ethanol-methanol and water-methanol mixtures. As the proportions of water and methanol in the sample change, the characteristic Raman spectra of molecules shift, and this phenomenon can be used to measure the concentrations of alcohols in water. d) Predicted concentrations (ethanol and isopropyl alcohol and the contaminants methanal and 1-propanol) based on a model derived by CDER researchers compared with actual concentrations for three container types (semi-transparent, transparent, opaque) and classification of hand sanitizers based on quantification by SORS–SVR
References
- FDA Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19) Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-temporary-policy-preparation-certain-alcohol-based-hand-sanitizer-products-during.
- World Health, O.; Safety, W. H. O. P., WHO guidelines on hand hygiene in health care. World Health Organization: Geneva, 2009.
- CDC Hand Hygiene Recommendations. https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html.
- FDA Updates on hand sanitizers consumers should not use (Accessed August 2021). https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-consumers-should-not-use.
- Overbeek DL, Watson CJ, Castaneda NR, Ganetsky M. A geographically distinct case of fatal methanol toxicity from ingestion of a contaminated hand sanitizer product during the COVID-19 pandemic. J Med Toxicol 2021, 17 (2), 218-221.
- Gupta N, Rodriguez JD, Yilmaz H. Through-container quantitative analysis of hand sanitizers using spatially offset Raman spectroscopy. Communications Chemistry 2021, 4 (1), 126.