FDA Investigation of Cronobacter Infections: Powdered Infant Formula (February 2022)
Do not use recalled Similac, Alimentum, or EleCare powdered infant formulas produced at Abbott Nutrition’s Sturgis, MI facility
If you want to check if your powdered formula is part of the recall, you can enter the product lot code on the bottom of your package on the company’s website.
If you have questions or need information about the recall, you can Submit Questions/Get Assistance.
If your infant is experiencing symptoms related to Cronobacter infection, such as poor feeding, irritability, temperature changes, jaundice, grunting breaths, or abnormal body movements; contact your health care provider to report their symptoms and receive immediate care.
To report an illness or adverse event, you can
- Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
- Complete an electronic Voluntary MedWatch form online.
- Complete a paper Voluntary MedWatch form that can be mailed to FDA.
The FDA, along with CDC and state and local partners are investigating consumer complaints and/or reports of infant illness related to products from Abbott Nutrition’s Sturgis, MI facility. All of the ill patients are reported to have consumed powdered infant formula produced from Abbott Nutrition’s Sturgis, MI facility.
To provide clarity about which products are included in the Abbott Nutrition recall, FDA is publishing a full list of recalled brands that have been included in the initial and expanded recall. Recalled products should no longer be available for sale, but if consumers have these products in their homes, they should check the lot code on the bottom of the package to determine if it is included in the recall.
The FDA is also providing additional information for parents and caregivers of infants receiving medical specialty infant formula and individuals using certain medical foods.
We understand that infant formula is the sole source of nutrition for many infants and is an essential product. FDA is continuing to work with Abbott Nutrition to better assess the impacts of the recall and understand production capacity at other Abbott facilities that produce some of the impacted brands. We are also working with Abbott Nutrition on safe resumption of production at the Sturgis, MI facility. FDA is continuing to investigate and will update this advisory should additional consumer safety information become available.
Recommendation
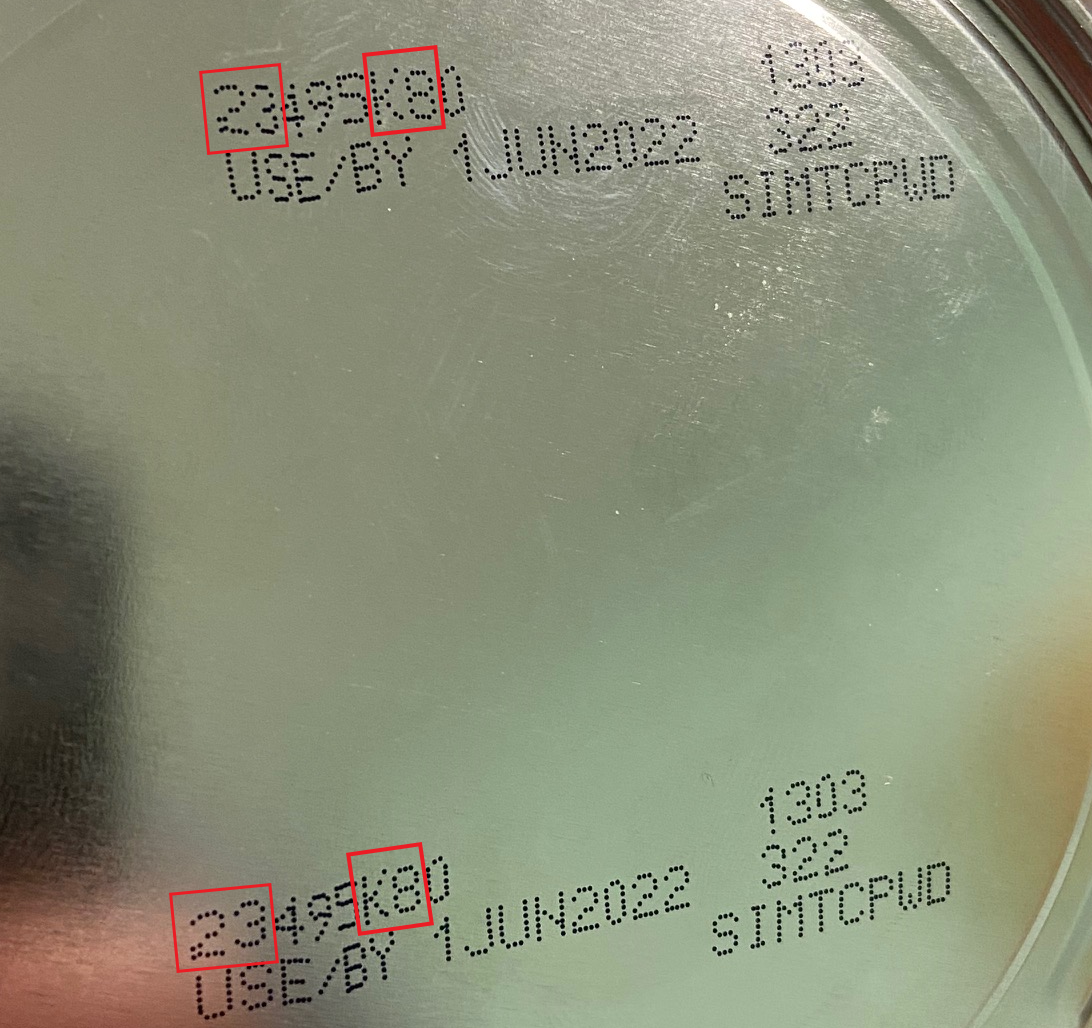
The FDA is advising consumers not to use recalled Similac, Alimentum, or EleCare powdered infant formulas. Recalled products can be identified by the 7 to 9 digit code and expiration date on the bottom of the package (see image below). Products are included in the recall if they have all three items below:
- the first two digits of the code are 22 through 37 and
- the code on the container contains K8, SH, or Z2, and
- the expiration date is 4-1-2022 (APR 2022) or later.
In addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code 27032K80 (can) / 27032K800 (case). At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) is the only type and lot of this specialty formula being recalled. Additional recall information for the initial recall is available on the FDA website. Parents can also enter their product lot code on the company’s website to check if it is part of the recall.
Additional Information for Parents and Caregivers:
The recalls do not include liquid formula products.
Parents and caregivers should never dilute infant formula and should not make or feed homemade infant formula to infants. Consumers should also avoid purchasing imported formula through online sales, as it has the potential to be counterfeit.
If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
If you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance. Also see:
More information on Cronobacter and infant formula is available on CDC’s website.
Recalled powdered infant formulas have the potential to be contaminated with Cronobacter, a bacterium that can cause severe foodborne illness primarily in infants. Cronobacter infections are rare but are especially high risk for newborn infants (see symptoms below).
Cronobacter bacteria can cause severe, life-threatening infections (sepsis) or meningitis (an inflammation of the membranes that protect the brain and spine). Symptoms of sepsis and meningitis may include poor feeding, irritability, temperature changes, jaundice (yellow skin and whites of the eyes), grunting breaths, and abnormal body movements. Cronobacter infection may also cause bowel damage and may spread through the blood to other parts of the body.
If your child is experiencing any of these symptoms, you should notify your child’s healthcare provider and seek medical care for your child immediately. Healthcare providers and health departments are encouraged to report any confirmed cases of Cronobacter sakazakii to CDC.
Product Images
Case Counts
Total Adverse Events: 4
Hospitalizations: 4
Reported Deaths: 2*
Illness Onset Date Range: 9/6/2021 – 1/4/2022
States with Adverse Events: MN (1), OH (2), TX (1)
Product Distribution: Nationwide and International
* Two deaths have been reported. Cronobacter infection may have contributed to the cause of death for both patients.
Useful Links
- U.S. Recall Announcement
- Expanded Abbott Nutrition Recall of Similac PM 60/40 Powdered Infant Formula
- Canadian Recall Warning
- Abbott Nutrition Recall Site
- CDC information on Cronobacter and infant formula
- CDC Investigation Notice: Cronobacter Illnesses Linked to Powdered Infant Formula
- Food Safety Tips for Consumers & Retailers During an Outbreak
- Who to Contact
- USDA FNS (WIC) Infant Formula Safety
- Toll-Free Numbers for WIC State Agencies
- USDA Contact Map
The following products are included in the recall if they have all three items below:
- the first two digits of the code are 22 through 37 and
- the code on the container contains K8, SH, or Z2, and
- the expiration date is 4-1-2022 (APR 2022) or later
Similac Products
Abbott Similac 360 Total Care Infant Formula with Iron
Abbott Similac Advance
Abbott Similac Advance Step-1
Abbott Similac Advance Step-2
Abbott Similac Advance Infant Formula with Iron
Abbott Similac Human Milk Fortifier
Abbott Similac Organic
Abbott Similac Organic with A2 Milk Infant Formula with Iron
Abbott Similac Organic with A2 Milk Toddler Drink
Abbott Similac Organic Toddler with A2 Milk Infant Formula with Iron
Abbott Similac Pro-Advance
Abbott Similac Pro-Advance Infant Formula with Iron
Abbott Similac Pro-Sensitive Infant Formula with Iron
Abbott Similac Pro-Total Comfort Infant Formula with Iron
Abbott Similac Sensitive
Abbott Similac Sensitive Infant Formula with Iron
Abbott Similac Sensitive Lactose Sensitivity
Abbott Similac for Supplementation
Abbott Similac For Spit Up Infant Formula with Iron
Abbott Similac Total Comfort
Abbott Similac Total Comfort Infant Formula with Iron
Abbott Similac EleCare HMO
Abbott Similac EleCare
Abbott EleCare Similac Gold
Abbott EleCare Similac
Abbott Similac PM 60/40 - only lot code 27032K80 (can) / 27032K800 (case)
Alimentum Products
Abbott Infant Formula Powder
Abbott Similac Alimentum
Abbott Similac Alimentum Allergies & Colic Hypoallergenic Infant Formula
Abbott Similac Alimentum with 2'-FL HMO
Abbott Similac Alimentum Eye Q Plus
Abbott Similac Alimentum HMO
Abbott Alimentum HMO
Abbott Similac Alimentum infant formula
Abbott Similac Alimentum Infant Formula with Iron
Abbott Similac Alimentum Toddler Drink
EleCare Products
Abbott EleCare
Abbott EleCare Amino Acid-Based Powder Infant Formula with Iron
Abbott EleCare Infant Formula Unflavoured
Abbott EleCare Junior Vanilla
Abbott EleCare Jr Similac Vanilla
Abbott EleCare Jr Amino Acid-Based Nutrition Powder Unflavored
Abbott EleCare Jr Amino Acid-Based Nutrition Powder Banana
Abbott EleCare Jr Amino Acid-Based Nutrition Powder Chocolate
Abbott EleCare Similac
Abbott EleCare Similac Gold
Abbott Similac EleCare HMO
Abbott Similac EleCare
EleCare LCP Hypoallergenic
Additional Information for Parents and Caregivers of Infants Receiving Medical Specialty Infant Formulas and Individuals Using Certain Medical Foods
The Abbott Nutrition facility that produces recalled infant formulas also produces metabolic and other medical specialty infant formulas for infants with inborn errors of metabolism and other medical needs, as well as medical foods. These products, with the exception of one lot of Abbott Similac PM 60/40, have not been recalled because the FDA has determined that the risk of not having these specialty products available could significantly worsen underlying medical conditions. For many of these patients, the risk of life-threatening adverse events from restricted access to these critically needed products is likely greater than the risk from consuming products that have been produced at the facility.
The FDA wants to be sure that parents and caregivers who use these specialty products are aware that there may be some risk of Cronobacter contamination. If possible, parents and caregivers should work with their medical provider to consider whether comparable products may be appropriate. If comparable alternative products are not available or appropriate, parents and caregivers should take extra care to follow the CDC’s updated advice for parents on how to reduce the risk of Cronobacter contamination of formula during preparation of powdered product, whether that contamination comes from the product itself or from other contamination sources in the home.
Glutarex-1
Glutarex-2
Cyclinex-1
Cyclinex-2
Hominex-1
Hominex-2
I-Valex-1
I-Valex-2
Ketonex-1
Ketonex-2
Phenex-1
Phenex-2
Phenex-2 Vanilla
Pro-Phree
Propimex-1
Propimex-2
ProViMin
Calcilo XD
Tyrex-1
Tyrex-2
Similac PM 60/40
It is important to note that these specialty infant formulas and medical foods are not sold in traditional retail stores. These products often require a prescription and are sold through specialty pharmacies and other specialty distribution channels such as medical product suppliers.
Parents and caregivers of infants and children using these products should contact their child’s health care providers if they have questions about the use of these products.
International Product Distribution Distribution for Recalled Products
The recall impacts Alimentum, EleCare, and Human Milk Fortifier for markets outside the U.S. No other Abbott Nutrition products distributed outside of the U.S. are affected by this recall. According to the firm, recalled products were distributed to the following countries/locations: Australia, Bahrain, Barbados, Bermuda, Canada, Chile, China, Colombia, Costa Rica, Dominican Republic, Ecuador, Egypt, Guam, Guatemala, Hong Kong, India, Indonesia, Israel, Jordan, Kuwait, Lebanon, Malaysia, Mexico, New Zealand, Oman, Peru, Puerto Rico, Qatar, Saudi Arabia, Singapore, South Africa, Sudan, Taiwan, Thailand, United Arab Emirates, United Kingdom, and Vietnam ANI South.
For additional information, international officials and customers can visit the company’s recall page for contact information.
Cronobacter Surveillance
Cronobacter infection surveillance is not handled the same way as infection with more common foodborne pathogens, such as Salmonella or E. coli O157:H7. Cronobacter is not nationally notifiable and not reportable except in one state, which means doctors and labs are not required to report cases to their health department. Because Cronobacter is not a nationally notifiable pathogen, FDA relies on consumer complaints of illness sent to the Agency and on health care providers informing FDA directly about infants with Cronobacter infections. In addition, because Cronobacter is not nationally notifiable, whole genome sequencing (WGS) is rarely performed on these isolates. To date, no outbreaks of Cronobacter have been detected using WGS.
When single cases of Cronobacter are reported, the FDA conducts a thorough review of each complaint, conducts sampling of products, and initiates inspections as appropriate. FDA collaborates with CDC, which has developed a detailed questionnaire specifically for Cronobacter infections that is often used by state health departments in instances of Cronobacter sakazakii infection.
Previous Updates
March 9, 2022
The Salmonella Newport illness previously included in this investigation of complaints and illnesses has been removed. In the early stages of this investigation, FDA included all consumer complaints of illness with exposure to products from the Sturgis, MI, facility. After further investigation, the FDA has determined that there is not enough information to definitively link this illness to powdered infant formula. CDC confirmed that this single Salmonella illness is not linked to an outbreak. The FDA and CDC are continuing to monitor for Salmonella cases and consumer complaints that may be related to this incident.
February 28, 2022
As of February 28, CDC has announced one additional illness of Cronobacter sakazakii with exposure to powdered infant formula produced at Abbott Nutrition’s Sturgis, MI facility. Cronobacter infection may have been a contributing cause of death for this patient. In total, this investigation includes four reports of Cronobacter sakazakii infections in infants (three from FDA complaints and one from a CDC case finding) and one complaint of a Salmonella Newport infection in an infant. All five (four Cronobacter infections and one Salmonella Newport infection) illnesses resulted in hospitalization and Cronobacter may have contributed to death in two patients.
The most recent patient was reported to have consumed Abbott Nutrition’s Similac PM 60/40 product with the lot code 27032K800 prior to Cronobacter sakazakii infection. FDA and CDC informed the firm of these findings and on February 28, 2022, Abbott Nutrition voluntarily recalled Similac PM 60/40 powdered infant formula with the lot code 27032K800. This is a specialty formula for certain infants who would benefit from lowered mineral intake and was not included in the previous recall. At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
This particular lot of Similac PM 60/40 was distributed to the U.S. and Israel. If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
We understand that infant formula is the sole source of nutrition for many infants and is an essential product. FDA is working with Abbott Nutrition to better assess the impacts of the recall and understand production capacity at other Abbott facilities that produce some of the impacted brands. We are also working with Abbott Nutrition on safe resumption of production at the Sturgis, MI facility. FDA is continuing to investigate and will update this advisory should additional consumer safety information become available.
February 25, 2022
As a result of the ongoing investigation, Abbott Nutrition has recalled certain powdered infant formula products and the FDA is advising consumers not to use recalled Similac, Alimentum, or EleCare powdered infant formulas produced in the Sturgis, MI facility.
We recognize that the Abbott Nutrition recall resulted in consumers seeking alternative brands or types of infant formula and that the recall has created new concerns about the availability of certain types of infant formula, particularly given the overall strains on supply chains experienced during the COVID-19 pandemic.
We understand that infant formula is the sole source of nutrition for many infants and is an essential product.
FDA is working with Abbott Nutrition to better assess the impacts of the recall and understand production capacity at other Abbott facilities that produce some of the impacted brands. We are also working with Abbott Nutrition on safe resumption of production at the Sturgis, MI facility. Throughout the pandemic, FDA has also been monitoring potential supply chain risks for this category of products and has been reaching out to infant formula manufacturers and their trade groups. As Abbott Nutrition was initiating its recall, FDA intensified this outreach to infant formula manufacturers to inquire about their capacity and potential impacts given this developing situation. We will continue discussion with Abbott Nutrition and other infant formula manufacturers and consider all tools available to support the supply of infant formula products.
We are also partnering with the United State Department of Agriculture’s Food and Nutrition Service (FNS) to monitor the impact of the recall on the WIC Program.
February 20, 2022
On 2/17/2022, Abbott Nutrition initiated a voluntary recall of certain powdered infant formulas. Products made at the Sturgis facility can be found across the United States and were likely exported to other countries/locations as well. Canadian health officials have also issued a recall warning.
February 17, 2022
The FDA, along with CDC and state and local partners are investigating four consumer complaints of infant illness related to products from Abbott Nutrition’s Sturgis, MI facility received from 9/6/2021 to 12/18/2021. All of the cases are reported to have consumed powdered infant formula (IF) produced from Abbott Nutrition’s Sturgis, MI facility. These complaints include three reports of Cronobacter sakazakii infections and one report of Salmonella Newport infection in infants. All four cases related to these complaints were hospitalized and Cronobacter may have contributed to a death in one case.
FDA has initiated an onsite inspection at the facility. Findings to date include several positive Cronobacter results from environmental samples taken by FDA, and adverse inspectional observations by FDA investigators. A review of the firm’s internal records also indicate environmental contamination with Cronobacter sakazakii and the firm’s destruction of product due to the presence of Cronobacter.
FDA is issuing this advisory to alert consumers to avoid purchasing or using certain powdered infant formula produced in the Sturgis, MI facility.
This is an ongoing investigation and the firm is working with the FDA to initiate a voluntary recall of potentially affected product. FDA is continuing to investigate and will update this advisory should additional consumer safety information become available.
Who to Contact
If you want to check if your powdered formula is part of the recall, you can enter the product lot code on the bottom of your package on the company’s website.
If you have questions or need information about the recall, you can Submit Questions/Get Assistance.
If your infant is experiencing symptoms related to Cronobacter infection, such as poor feeding, irritability, temperature changes, jaundice, grunting breaths, or abnormal body movements; contact your health care provider to report their symptoms and receive immediate care.
To report an illness or adverse event, you can
- Call an FDA Consumer Complaint Coordinator if you wish to speak directly to a person about your problem.
- Complete an electronic Voluntary MedWatch form online.
- Complete a paper Voluntary MedWatch form that can be mailed to FDA.