CDER Continues to Make Rare Diseases a Priority with Drug Approvals and Programming to Speed Therapeutic Development

By: Patrizia Cavazzoni, M.D., Director, Center for Drug Evaluation and Research
A rare disease is any disease that affects less than 200,000 people in the U.S. Drug development for the approximately 7,000 rare diseases can be complex for many reasons. Challenges exist with using well-established trial designs. Selecting endpoints (outcome measures) can be difficult if there is a limited understanding of the natural history of the disease. Because of the small patient populations, there may not be enough people available to participate in rare disease clinical trials. For these and other reasons, many of these diseases have few or no available treatments.
The FDA’s Center for Drug Evaluation and Research (CDER) has teams and programs that are dedicated to overcoming these hurdles and facilitating the development of therapies for the more than 30 million people living with a rare disease in the U.S. The combination of government incentives and scientific advancements has fueled extraordinary development of drugs for rare diseases.
CDER’s Approvals for Rare Diseases
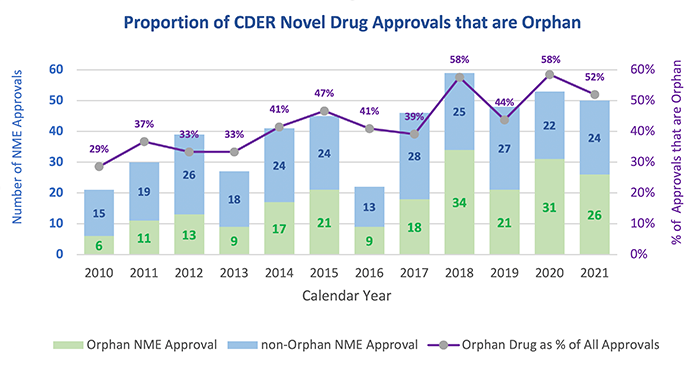
Over the past decade or so, we have seen an upward trajectory in the percentage of drugs approved to treat rare or “orphan” diseases. Last year, 26 of CDER’s 50 novel drug approvals — more than half — were for orphan diseases.
And only two months into 2022, we have approved four new drugs for people with rare diseases in the areas of oncology and hematology. These drugs include:
- The first therapy for uveal melanoma (a rare cancer that develops in a part of the eye called the uvea) that has spread to other parts of the body or cannot be surgically removed.
- The first therapy to decrease the need for red blood cell transfusion due to hemolysis (red blood cell destruction) in adults with cold agglutinin disease, a rare type of anemia.
- The first therapy to treat anemia in adults with pyruvate kinase deficiency, an inherited disorder that causes premature red blood cell destruction.
- A drug for adults with intermediate or high-risk primary or secondary myelofibrosis, a rare bone marrow disorder, who have low platelet (blood clotting cells) levels.
CDER’s Efforts to Support Rare Disease Drug Development
Despite this progress, the vast majority of rare diseases still do not have approved therapies. We must do more work to address the needs of patients with rare diseases and their families.
To that end, CDER has multiple efforts underway to support rare disease drug development. Because we believe that data sharing is critical to advancing drug development, particularly for rare diseases, we are continuing important work with the Critical Path Institute by developing the Rare Disease Cures Accelerator-Data and Analytics Platform. The platform provides a database and hub designed to promote the secure sharing of existing patient-level data and encourage the standardization of new data collection. It allows authorized users to access patient-level clinical data to better understand disease progression and disease heterogeneity (or differences among people with the same disease) across rare disease patient populations. In turn, this can inform trial design, selection of endpoints, and other important considerations for drug development.
Also, as part of the Standard Core Clinical Outcome Assessment Grant Program, CDER is working with grantees to develop publicly available core sets of clinical outcome assessments that may be used as endpoints in clinical trials. Some assessments developed through this project include physical function, pain in infants and young children, and communication in children with neurodevelopmental disorders. We are also working with investigators from the University of Michigan to develop innovative trial designs for rare diseases with small populations.
CDER is optimistic about the future of supporting rare disease drug development and we are looking forward to continuing our work — together with patients, advocacy groups, industry, and other partners — to address the significant unmet needs of patients and families living with rare diseases. As we commemorate the FDA’s Rare Disease Day 2022, we recognize these patients and the people working alongside them to make more treatments available.