From Our Perspective | A Two-Part Series: Risk Evaluation and Mitigation Strategies (REMS) Program
Part 1 - Program Participants and Roles
By: Cynthia LaCivita, PharmD, Division Director, Division of Risk Management, Office of Surveillance and Epidemiology, Centers for Drug Evaluation and Research and Laura Zendel PharmD, Associate Director for REMS Design and Evaluation, Division of Risk Management, Office of Surveillance and Epidemiology, Centers for Drug Evaluation and Research
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program that the U.S. Food and Drug Administration can require for a certain medication with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS use risk minimization tools to reinforce behaviors and actions that support the safe use of that medication. A REMS can be required pre-approval or postapproval if new safety information is identified. It is important for health care professionals, patients, and all others whom REMS affect to be aware that these strategies can provide safe access for patients to certain drugs with serious risks that may otherwise not be approved and available on the market.
While all medications have FDA-approved labeling addressing risks, only certain medications require a REMS. REMS do not apply to nonprescription drugs. The law requires that when a brand name drug has a REMS, any generics for this drug must also have a REMS. The brand name manufacturer and the generic version’s manufacturer often jointly develop and implement a REMS (i.e., a shared system REMS) once generic versions of the drug become available. For a small number of REMS programs, the brand name drug and the generic drug each have separate comparable REMS, but both have the same goals and the same REMS requirements.
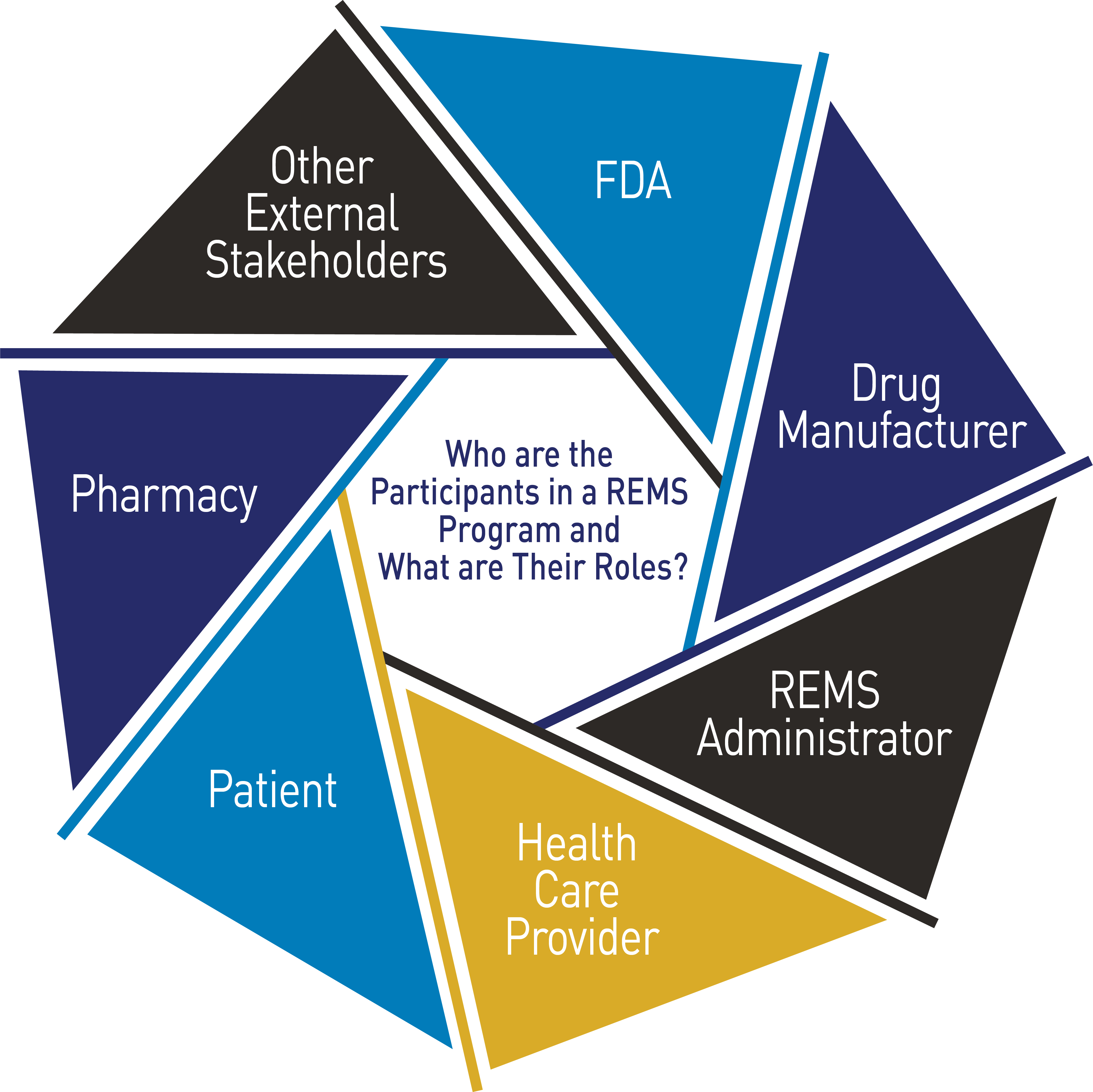
There may be several participants within each REMS program, all with different and unique roles and responsibilities. The specific requirements for each participant in the REMS are described in detail in the REMS documents and REMS materials.
FDA’s Role
Our REMS experts apply a number of factors in determining if a REMS is necessary, including (but not limited to) the seriousness of any known or potential adverse events that may be related to the drug, the expected benefit of the drug with respect to the disease or condition it is being used to treat, and whether additional interventions beyond FDA-approved labeling are necessary to ensure that the drug’s benefits outweigh its risks.
If we determine that a REMS is necessary, our experts review the REMS to be sure it describes the goals of the program and the specific requirements that are necessary to ensure the safe use of the drug. After a REMS has been approved, FDA can enforce effective implementation of the REMS by applicants. We review the manufacturer’s REMS assessment reports, determine if the REMS is meeting its goals, and if the REMS goals, elements or requirements, or assessment plan should be modified. FDA has the authority to require the drug manufacturer to modify the REMS to address new safety information or to minimize the burden on the health care delivery system of complying with the REMS.
Drug Manufacturer’s Role
While FDA determines if a REMS is necessary, the drug manufacturer is responsible for developing and implementing the REMS program. The drug manufacturer is also responsible for assessing the effectiveness of the REMS in meeting its risk mitigation goals and ensuring that the program is being implemented as intended. Following the REMS approvals, drug manufacturers are required to submit reports of the REMS assessments to FDA that includes analysis, findings, and conclusions related to whether the REMS is meeting its goals and what, if any, modification may be needed.
Health Care Professional’s Role
The requirements for health care professionals who prescribe a drug with REMS or who are involved in the care of patients who are prescribed a drug with REMS will vary for each REMS. For most REMS, health care professionals will receive communications about the drug and the REMS from the drug manufacturers. REMS with Elements to Assure Safe Use (ETASU) may have other requirements for health care professionals, such as enrollment in the REMS, completion of training, documentation of counseling of patients, enrollment of patients in the REMS, patient monitoring, and/or documentation of safe use conditions.
Patient’s Role
Patients play an essential role in REMS. The patient role varies and depends on the requirements of the REMS. Patients may receive specific information or counseling about a serious risk associated with the medication, what action they need to take to mitigate a serious risk, and what symptoms they need to watch for and report to their health care professional. For some medications, patients must sign a form acknowledging that they understand those risks before starting the medication. For other medications, patients need to undergo lab testing. Patients may be required to be enrolled in the REMS to ensure monitoring is conducted or to document if a specific adverse event occurs while they are taking the medication.
Pharmacy’s Role
Pharmacists and other practitioners who dispense medicines play a key role in ensuring that products with serious risks requiring REMS are dispensed and used safely.
The requirements for pharmacists will vary for each REMS and by setting (i.e., retail or inpatient pharmacy). For most REMS, pharmacists and other dispensers will receive communications about the drug and the REMS from the drug manufacturers. Certain REMS may require pharmacies or other health care settings to become certified to dispense the medication with REMS. Individual pharmacists may be required to complete training, verify safe use conditions (i.e., verifying required laboratory monitoring or that a patient or prescriber is enrolled in the REMS), counsel patients, and/or provide the patient with educational materials or a Medication Guide. REMS with ETASU often require pharmacists to receive authorization from the REMS Administrator (drug company or a vendor who implements the REMS program) before dispensing the medication to the patient.
Other External Stakeholders’ Role
In addition to the stakeholders mentioned above, there are other stakeholders that help to carry out certain REMS requirements. For example, wholesaler and distributors play an important role in maintaining the integrity of a REMS that includes restricted distribution by only distributing product to entities that are certified in the REMS. Prescribers may delegate some of the administrative tasks to certain office or nursing staff within their practice. Health care professionals, including pharmacy staff, may be required to follow policies and procedures that were instituted by the authorized representatives in those entities that are certified in the REMS.
Stay tuned for Part 2 of this REMS From Our Perspective series on challenges and opportunities for certain REMS programs.