Medical Gloves for COVID-19

Medical gloves are used by health care personnel to prevent the spread of micro-organisms that may potentially cause infection or illness. Medical gloves are disposable and include patient examination gloves and surgeon’s gloves.
The FDA continues to find and stop those selling unsafe and ineffective products by investigating, examining and reviewing medical products, both at ports of entry and within the U.S. market, to help ensure the safety of our national supply chain.
To help expand the availability of medical gloves during the COVID-19 public health emergency, the FDA is providing regulatory flexibility under certain circumstances, as described in the enforcement policy for gloves during the COVID-19 public health emergency. Below are answers to frequently asked questions about medical gloves.
On this page:
- The Basics on Medical Gloves and COVID-19
- Manufacturing Medical Gloves During COVID-19
- Importing or Purchasing Medical Gloves During COVID-19
- Reporting Shortages or Problems with Medical Gloves
Related page:
- Registration and Listing of Medical Devices during the COVID-19 Pandemic: Describes registration and listing requirements for manufacturers and importers of gloves
The Basics on Medical Gloves and COVID-19
Q: Do medical gloves provide protection from coronavirus?
A: Medical gloves are intended to provide broad barrier protection. Please see current recommendations from Center for Disease Control and Prevention (CDC) for patients with suspected or confirmed COVID-19. At this time, FDA has not cleared, approved, or authorized any medical gloves for specific protection against the virus that causes COVID-19 or prevention of COVID-19 infection.
Q: What is the FDA’s policy on medical gloves during and after COVID-19?
A: Most medical gloves are subject to premarket notification under section 510(k) of the Federal Food, Drug, and Cosmetic Act (FD&C Act). For more information, see Premarket Notification 510(k). The FDA issued a guidance document entitled Enforcement Policy for Gowns, Other Apparel, and Gloves During the Coronavirus Disease (COVID-19) Public Health Emergency to provide a policy to help expand the availability of these items for health care professionals. This document includes the FDA’s policies regarding surgeon’s and patient examination gloves during the COVID-19 pandemic. This Enforcement Policy remains in effect.
Q: What types of medical gloves does the FDA’s policy apply to?
A: The FDA’s policy applies to these types of medical gloves, which are also listed in Table 2 of the policy:
| Glove Type | Product Code |
|---|---|
| Patient Examination Glove | FMC |
| Latex Patient Examination Glove | LYY |
| Polymer Patient Examination Glove | LZA |
| Finger Cot | LZB |
| Vinyl Patient Examination Glove | LYZ |
| Powder-Free Guayle Rubber Examination Glove | OIG |
| Powder-Free Polychloroprene Patient Examination Glove | OPC |
| Radiation Attenuating Medical Glove | OPH |
| Specialty Patient Examination Glove | LZC |
| Surgeon’s Gloves | KGO |
| Powder-Free Non-Natural Rubber Latex Surgeon’s Gloves | OPA |
The FDA’s policy does not apply to powdered surgeon’s gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon’s glove, which are banned devices and are not eligible to be distributed in the United States.
Q: Since the FDA has removed medical gloves from the device shortage list, will the related enforcement policy be revoked?
A: The removal of medical gloves (product codes LYY, LYZ, LZA, and LZC) from the device shortages list does not impact the existing Enforcement Policy for Gowns, Other Apparel, and Gloves During the Coronavirus Disease (COVID-19) Public Health Emergency. The policy remains in effect at this time.
Q: Which medical gloves are FDA-cleared?
A: To identify FDA-cleared medical gloves, search the 510(k) Premarket Notification database using the product codes for medical gloves listed in the Q: What types of medical gloves does the FDA’s policy apply to?
Manufacturing Medical Gloves During COVID-19
Q: I’m new to working with the FDA. What do I need to do if I’m interested in manufacturing medical gloves during COVID-19?
A: Those interested in manufacturing medical gloves should consider submitting a premarket notification under section 510(k) of the FD&C Act. For more information, see Premarket Notification 510(k). During the COVID-19 public health emergency, the FDA has not expected manufacturers of medical gloves to submit notification to the FDA before they begin marketing their product or to comply with certain regulatory requirements where the medical gloves do not create an undue risk in light of the pandemic as outlined in the FDA’s policy for gloves during the COVID-19 public health emergency.
Under the policy, the FDA believes patient examination gloves do not create such an undue risk where:
- The gloves include labeling that:
- Accurately describes the product as an “unpowdered glove” (as opposed to a “surgeon’s glove”);
- Accurately describes of the product’s sterility status such as, non-sterile);
- Does not claim that the product is latex-free or otherwise free of a specific material;
- Includes a list of the body contacting materials; and
- Includes recommendations and general statements that would reduce the risk of use. For example:
- A statement that the gloves have not been cleared by FDA.
- A recommendation against using when FDA-cleared gloves are available.
- A recommendation against using in surgical settings.
- The product is not intended for any use that would create an undue risk. For example, the labeling does not include uses with chemotherapy drugs, fentanyl, and other opioids, for allergy or dermatitis prevention, for antimicrobial or antiviral protection, or for infection prevention or reduction.
Under the policy, the FDA believes that surgeon’s gloves do not create an undue risk where:
- The gloves meet the FDA-recognized standard described in ASTM D3577 and have been manufactured in compliance with good manufacturing practices.
- The gloves include labeling that:
- Accurately describes product as being unpowdered;
- Accurately describes the product’s sterility status (that is, sterile) and the sterilization method used;
- Does not claim that the product is latex-free or otherwise free of a specific material;
- Includes a list of the body contacting materials; and
- Includes recommendations and general statements that would reduce the risk of use. For example:
- A statement that the gloves have not been cleared by FDA.
- A recommendation against use when FDA-cleared surgeon’s gloves are available.
- The product is not intended for any use that would create an undue risk. For example, the labeling does not include uses with chemotherapy drugs, fentanyl, and other opioids, for allergy or dermatitis prevention, for antimicrobial or antiviral protection, or for infection prevention or reduction.
Q: What materials can I use to make medical gloves?
A. The FDA does not have a list of materials used to make medical gloves. However, in our experience, medical gloves are most often made of different materials, including nitrile, polyvinyl chloride (PVC), polyurethane, and neoprene.
Please be aware that powdered medical gloves are banned pursuant to the Final Rule on Banned Devices for powdered surgeon's gloves, powdered patient examination gloves, and absorbable powder for lubricating a surgeon's glove.
Q: Does the FDA have standards to follow to manufacture medical gloves?
A: The American Society of Testing and Materials provides information regarding standards for various personal protection equipment. The FDA had also previously issued the Medical Glove Guidance Manual.
Q: Does the FDA have recommendations on patterns or templates to manufacture gloves?
A: The FDA does not have patterns or templates for medical gloves to share with the public or recommendations regarding materials beyond those listed in the standards referred to in the previous answer.
Q: Whom do I contact if I have questions about manufacturing or distributing medical gloves?
A: If you have read the Enforcement Policy guidance document as well as the information on this page and you have questions, send an email to [email protected].
Importing or Purchasing Medical Gloves During COVID-19
Q: I’m having trouble importing medical gloves into the United States. What do I need to do?
A: Check the Import Alert 80-04 “Surveillance and Detention Without Physical Examination of Surgeon's and Patient Examination Gloves” and Import Alert 89-08 "Detention Without Physical Examination of Devices without Approved PMA's or IDE's and Other Devices Not Substantially Equivalent or Without a 510(k)" to make sure the medical gloves you would like to import are not subject to the import alert.
Import alerts inform the FDA’s field staff and the public that the agency has enough evidence to allow for Detention without Physical Examination (DWPE) of products that appear to be in violation of the FDA’s laws and regulations. These violations could be related to the product, manufacturer, shipper, and/or other information.
In order to avoid delays of legitimate shipments, review the Importing COVID-19 Supplies and FDA’s instructions to importers sent by U.S. Customs and Border Protection for important information surrounding importing products, including gloves, to ensure that the proper documentation is submitted at the time of entry. The FDA has been ready and available to engage with importers to minimize disruptions during the importing process. If you have any specific import questions, you may email [email protected].
Q: Do I need an Emergency Use Authorization to import medical gloves?
A: No, you do not need to request that the FDA issue an Emergency Use Authorization (EUA) to import medical gloves.
Q: I would like to purchase medical gloves for health care workers. Where can I find them?
A: The FDA does not maintain a list of medical glove suppliers. However, to identify FDA-cleared medical gloves, search the 510(k) Premarket Notification database using the product codes for medical gloves described in Q: What types of medical gloves does the FDA’s policy apply to?. Note that the FDA issued a guidance document entitled Enforcement Policy for Gowns, Other Apparel, and Gloves During the Coronavirus Disease (COVID-19) Public Health Emergency to provide a policy to help expand the availability of these items for health care professionals. This document includes the FDA’s policies regarding surgeon’s and patient examination gloves during the COVID-19 pandemic.
If you are a health care facility, check with your supplier, distributor, or your local health department. You may also want to check with the Association for Health Care Resource & Materials Management (AHRMM) of the American Hospital Association, which maintains the AHRMM Novel Coronavirus (COVID-19) Update on health care supply chain issues.
Q: I would like to purchase medical gloves. Do I need to ask the supplier for a certificate?
A: The FDA does not issue any kind of certification to demonstrate that a manufacturer is in compliance with the FDA’s requirements. The FDA recommends that manufacturers follow recognized standards for testing of medical gloves. You may consider asking for that information when purchasing medical gloves.
Q: How can I tell if the medical gloves I want to purchase are counterfeit or fraudulent?
A: The FDA does not have an exhaustive list of all counterfeit or fraudulent products. The FDA issues Import Alerts for products that appear to be in violation of the FDA's laws and regulations, as described in Q: I’m having trouble importing medical gloves into the United States. What do I need to do?
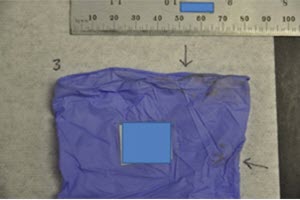
If you have purchased any medical gloves that appear to be fraudulent, such as those described and pictured below, please report it to the FDA at [email protected]. Signs that medical gloves may be fraudulent include, but are not limited to, signs that the medical gloves:
- Are visibly soiled
- Are a different color, size and materials
- Contain any powder particulates or any visible contaminants (powdered gloves are banned in the US)
- Look discolored, such as too dark or faded
- Leak any colorants on to hands when touched or used
- Appear to have been used
- Include certain labeling that the FDA has not cleared or approved, such as:
- Antimicrobial or antiviral protection, including statements that gloves contain antimicrobials such as chlorhexidine, silver, or zinc
- Infection prevention or reduction
- Allergy or dermatitis prevention
- Not provided in English, with the exception of products distributed solely within Puerto Rico or a U.S. territory where the predominant language is other than English.
- In these instances, the predominant language may be substituted for English (as described in Labeling Regulatory Requirements for Medical Devices).
- Include labeling that is contradictory to the gloves in the package, such as labeling that indicates:
- a vinyl patient examination glove, while the gloves in the box are nitrile gloves.
- the gloves are blue, but the gloves in the box are white.
- the gloves are powder-free, while the gloves in the box appear powdered
- May cause itching, irritation, or other skin reaction
- Easily develop holes or tears
Soiled gloves
Soiled Gloves
Soiled gloves
Soiled gloves
Discolored gloves
Discolored gloves
Discolored gloves
Torn gloves
Reporting Shortages or Problems with Medical Gloves
Q: How do I report a shortage of medical gloves?
A: Contact the FDA about a medical device supply chain issue.
Q: How do I report a problem with medical gloves?
A: The FDA encourages health care providers to report any product problems, adverse events or suspected adverse events experienced with medical gloves.
- Voluntary reports can be submitted through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
- Device manufacturers and user organizations must comply with the applicable Medical Device Reporting (MDR) regulations.
- Health care personnel employed by organizations that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their organizations.