The Hunt for a Blinding Bacterium
By: Erin Peabody
How FDA scientists rushed to i.d. the culprit behind dangerous eye drops, to halt further harm to the public.
Shifty, tough, and hard to predict, superbugs pose a serious threat to public health.
extra-long flagellum to swim, swarm, and spread
infection inside their hosts.
These super strains of bacteria, viruses, and fungi can cause devastating infections, known for their genetic ability to turn on a dime, so to speak, picking up new defenses and resistances that can render even the most potent antibiotics and other drugs useless.
Among the worst of these: the bacterium Pseudomonas aeruginosa (pronounced soo-duh-mow-nuhs eh-roo-jih-no-suh). It’s been called “audacious” and “a nightmare” by scientists, a group not prone to theatrics, and now ranks as the second most dangerous drug-resistant microbe out there, according to the World Health Organization, who calls for the development of new drugs that can go toe-to-toe with it immediately.
Adding to its notoriety, bacterium became a household name last year when federal scientists discovered it was the likely source of contamination in several brands of eye drops.
The products, produced in unsanitary conditions in a facility in India, as later confirmed by FDA investigators, had caused serious harm to some consumers, including eye infections, vision loss, and, in a few instances, death.
Getting It Right
“It’s very, very scary,” says microbiologist Crystal Nevins about the challenges superbugs present, including their capacity for harm.
Nevins would know. The FDA scientist and her team of microbiologists and analysts, based out of the agency’s medical products laboratory in Jamaica, New York, were practically obsessed with Pseudomonas all of last year.
Theirs was one of three agency labs dedicated to finding answers during the eye drops outbreak—namely to confirm if the vexing superbug was, in fact, the source of contamination.
If the scientists made a mistake, then an already bad situation could become worse. The wrong treatment, or drug, could be administered to infected patients. Other costly efforts to contain the outbreak could be wasted.
And most dangerous? Pointing the finger at the wrong bacteria would leave a potential killer on the loose.
“There was a lot of pressure, for sure,” Nevins said about the urgency of the situation. “We wanted to deliver results as quickly as we could, but we also knew, we had to get it right.”
Analysis Under Pressure
Rigorous analysis is needed to bring an outbreak to an end; it’s also the gold standard to which all regulatory scientists are bound. Their science must hold up in the lab, and—at the same time—prove unassailable in court.
Scrutiny is just another part of the job, says Nevins’ colleague, microbiologist Philip Istafanos, a 34-year veteran of the agency, who once spent nearly four hours on a witness stand defending his scientific methods, which, in 2012, were used to help solve a deadly fungal infection outbreak and provide government prosecutors the evidence needed to put a fraudulent pharmacist behind bars.
“Regulatory scientists have to do it by the book, for many reasons,” Istafanos explains. “Ultimately, it’s about doing all we can, so the public can be confident in the drugs and medical products they need.”
For the eyedrops outbreak, that first important step was to get the eye drop samples arriving at the lab in an optimal state for study.
The clear liquid drops we use to rewet our eyes might seem pretty simple, from a chemical standpoint, but, as Nevins points out, “they’re a difficult matrix to work with.”
Eye drops contain a cellulose ingredient that, under analysis, can gel up and complicate further testing. This meant all samples would need to be filtered first—an unavoidable step, but one Nevins and her team also exploited: They used a filtering system that would allow them to manipulate the solution under airtight containment, inside one of the cleanest places on Earth.
Sterility is Key
FDA laboratory cleanrooms, like the one in Queens—where scientists regularly test drugs, medical devices, and living tissue implants to ensure that they’re safe and sterile for patients—demand a kind of hyper-hygiene that most of us can’t imagine.
Before analysts can enter these highly controlled spaces, they must don gowns, hoods, gloves, goggles, face masks, and booties. HEPA filters are checked frequently, as are sticky mats on the floor, to grab any stray particles that shoe coverings don’t stop.
The truth is, in a lab environment, humans must be regarded as the large shuffling hazards they really are: sources of shedding hair, skin flakes, saliva droplets, food bits, and more. If any of these particles are allowed to reach a sample, then accuracy is compromised.
Istafanos says that, despite years of working in this space, he’s always on high alert in the cleanroom. “Every time I go in, I treat it like it’s my first time there.”
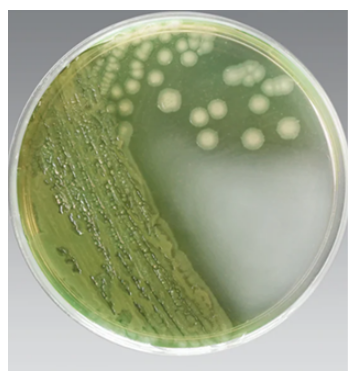
when in the presence of the antiseptic cetrimide.
This color reveal helps FDA scientists positively i.d.
the pathogen when they’re working to solve
frightening outbreaks.
Shrouded head to toe in disposable clothing, the microbiologist brought this sharp attention to the eye drops scare, when he spent days at a time in the Queens-based cleanroom, prepping hundreds of samples for further analysis.
No Assumptions
While Pseudomonas was the microbiologists’ number one suspect, they couldn’t rule out the possibility that other pathogens, or microbes capable of causing infection or disease, in the eye drops might be contributing to infections and injuries.
“We couldn’t make any assumptions,” says Nevins. “We had to look for everything.”
Ironically, this meant the scientists would need to coax out of hiding any microbes—bacteria, and fungi too—that might be lurking inside the tainted eye drops. The team supplied a broad range of nutrients, plus two different incubation temperatures to help the process along.
Then, they waited. It was frustrating, to be sure. Depending on the level of contamination, it could take several days before any microbes stirred to life.
Regardless, as the clock ticked, Nevins says her team watched the incubating cultures “like a hawk.” As soon as a culture turned cloudy—a sign of microbial growth—the analysts could move on to the next step: to subculture, or pinpoint, the pathogen at the heart of the contamination.
They wouldn’t have to wait long. Within hours, the media inside several of the testing canisters turned turbid. It was time to finally pull any hiding Pseudomonas out the shadows, something the team would do with the help of a special agar plate treated with, of all things, cetrimide.
Cetrimide is an antiseptic best known for killing bacteria (it’s often found in wound-cleaning topicals), but what it conjures in the presence of Pseudomonas aeruginosa is surprisingly beautiful, and—most critical during an outbreak—conclusive.
Significant Contamination
“The cetrimide agar plate turns this interesting green color if Pseudomonas aeruginosa is there,” explains Istafanos.
While the shifty bacterium largely confounds scientists, the distinctive blue-green pigment it produces under certain conditions, called pyocyanin, gives it away nearly every time.
The New York lab witnessed this color reveal numerous times during its weeks-long analyses.
“I was pretty astonished,” says Istafanos. “There was one instance when we ran the usual ten tests on a single eye drop sample, and every one of those ten came back positive for Pseudomonas.”
The evidence was clear. The eye drops causing harm to consumers were highly contaminated, tainted by primary suspect and superbug Pseudomonas aeruginosa.
The lab promptly relayed all findings to FDA officials, while also collaborating with the agency’s Irvine Medical Products Laboratory, which had also tested hundreds of eye drop samples, and its Winchester Engineering Analytical Center, known as WEAC, located just outside of Boston.
With whole genome sequencing capability, FDA’s WEAC scientists confirmed what the New York lab determined. They also revealed a little more: that the dangerously dirty eye drops harming consumers harbored a new, never-before-seen strain of Pseudomonas.
With scientific certainty in hand, recalls were initiated, and boxes of eye drops were pulled from store shelves. Doctors had the definitive information needed to optimally treat their affected patients.patients.
FDA scientists don’t always learn about the real and positive effects of their work, says Nevins, especially when it’s a crisis averted, with nothing to report in the news. But in this case, she and her team did.
“It brings a lot of satisfaction to know we were part of something important,” she says, “that we helped get dangerous products out of commerce and save lives.”
Istafanos agrees. “Thank God there are few instances like this one. But as regulatory scientists serving the public, that’s what we’re here for.”
