Drug Trials Snapshots: CAMZYOS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the CAMZYOS Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
CAMZYOS (mavacamten)
(kam zye’ ose)
Myokardia Inc
Approval date: April 28, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CAMZYOS is a cardiac myosin inhibitor used for the treatment of symptomatic New York Heart Association (NYHA) Class II to III obstructive hypertrophic cardiomyopathy (HCM) in adults to improve functional capacity and symptoms.
How is this drug used?
CAMZYOS is a capsule that is taken once a day.
Who participated in the clinical trials?
The FDA approved CAMZYOS based on evidence from a clinical trial of 251 patients with symptomatic obstructive HCM. The trial was conducted in 68 sites in the European Union, the United States, Israel, and the United Kingdom.
What are the benefits of this drug?
CAMZYOS may improve your symptoms and your ability to be active.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary composite functional endpoint, assessed at 30 weeks, was defined as the proportion of patients who achieved either improvement of peak oxygen consumption (pVO2) by ≥1.5 mL/kg/min plus improvement in NYHA class by at least one, or improvement of pVO2 by ≥3.0 mL/kg/min plus no worsening in NYHA class.
A greater proportion of subjects met the primary endpoint at Week 30 in the CAMZYOS group compared to the placebo group (37% versus 17%, respectively, p=0.0005).
Table 1. Primary Endpoint at Week 30
| Parameter | CAMZYOS N=123 |
Placebo N=128 |
Difference (95% CI) |
P-value |
|---|---|---|---|---|
| Total responders | 45 (37%) | 22 (17%) | 19% (9, 30) | 0.0005 |
| Change from baseline pVO2 ≥1.5 mL/kg/min and decreased NYHA | 41 (33%) | 18 (14%) | 19% (9, 30) | |
| Change from baseline pVO2 ≥3 mL/kg/min and NYHA not increased | 29 (23%) | 14 (11%) | 13% (3, 22) |
Source: CAMZYOS Prescribing information
Abbreviations: CI, confidence interval; NYHA, New York Heart Association; pVO2, peak oxygen consumption
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The effect of CAMZYOS was similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how CAMZYOS worked among races could not be determined.
- Age: CAMZYOS worked similarly across all age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 summarizes how well CAMZYOS worked in the trial among sex and age groups.
Table 2. Effect of CAMZYOS on Improving Functional Capacity and Symptoms (Primary Endpoint at Week 30), by Sex and Age Subgroups
| Subgroup | Achieved Composite Functional Endpoint n (%) |
Difference in Proportion of Subjects Who Achieved the Composite Function Endpoint (95% CI)* | |
|---|---|---|---|
| CAMZYOS N=123 |
Placebo N=128 |
||
| Sex | |||
| Male | 26.0 (39.4) | 17.0 (20.5) | 19.5 (5.9, 33.0) |
| Female | 19.0 (33.3) | 5.0 (11.1) | 21.8 (7.8, 35.9) |
| Age, years | |||
| ≤49 | 10.0 (37.0) | 6.0 (24.0) | 16.9 (-2.3, 36.2) |
| 50 to 64 | 21.0 (41.2) | 13.0 (20.6) | 20.5 (6.4, 34.6) |
| ≥65 | 14.0 (31.1) | 3.0 (7.5) | 22.4 (8.6, 36.1) |
Source: Adapted from FDA Review
* Treatment differences and credible intervals may not match values of (CAMZYOS - Placebo) since estimates include relevance of outcomes from other subgroups
Abbreviations: CI, confidence interval
What are the possible side effects?
CAMZYOS may cause serious side effects, including heart failure where the heart cannot pump with enough force. This is a serious condition that can lead to death. The risk of heart failure is also increased when CAMZYOS is taken with medications that can lower the rate of its metabolism, thereby causing a buildup of the drug.
Because of the serious risk of heart failure, CAMZYOS is only available through a restricted program called the CAMZYOS Risk Evaluation and Mitigation Strategy (REMS) Program.
The most common side effects of CAMZYOS include dizziness and fainting (syncope).
What are the possible side effects (results of trials used to assess safety)?
Adverse reactions occurring in >5% of patients and more commonly with CAMZYOS than placebo in the trial were dizziness (27% versus 18%) and syncope (6% versus 2%).
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in side effects between races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 3 summarizes the side effect of dizziness by demographic subgroup. Subgroup analysis for the side effect of syncope was not conducted due to the overall small number of the events reported in the clinical trial.
Table 3. Subgroup Analysis for Dizziness AEs
| Demographic Parameter | CAMZYOS N=123 |
Placebo N=128 |
|---|---|---|
| Overall | 33/123 (27%) | 23/128 (18%) |
| Sex | ||
| Female | 13/57 (23%) | 8/45 (18%) |
| Male | 20/66 (30%) | 15/83 (18%) |
| Age, years | ||
| <65 | 20/78 (26%) | 17/88 (19%) |
| ≥65 | 13/45 (29%) | 6/40(15%) |
Source: Adapted from FDA review
Abbreviations: AE, adverse event
DEMOGRAPHICS SNAPSHOT
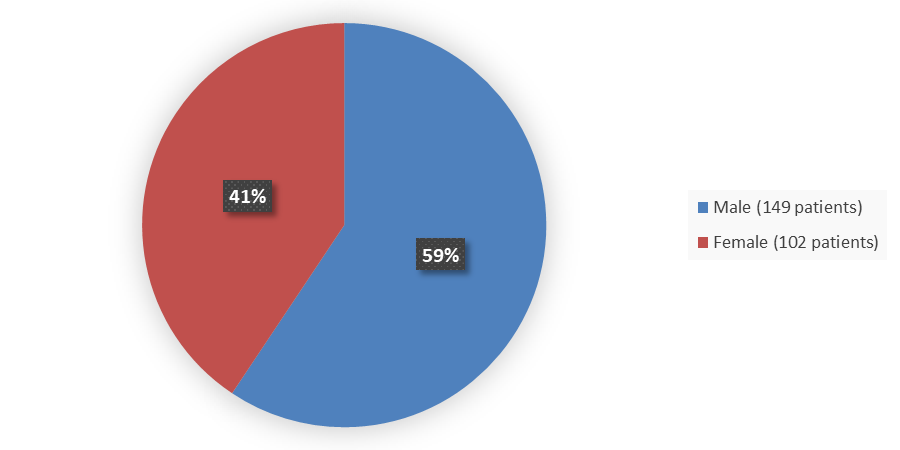
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy and side effects of CAMZYOS.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Clinical review
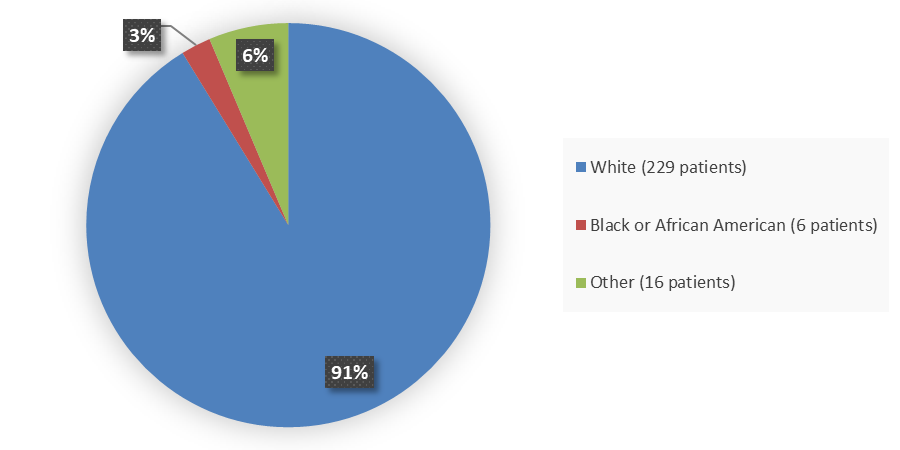
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy and side effects of CAMZYOS.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Clinical review
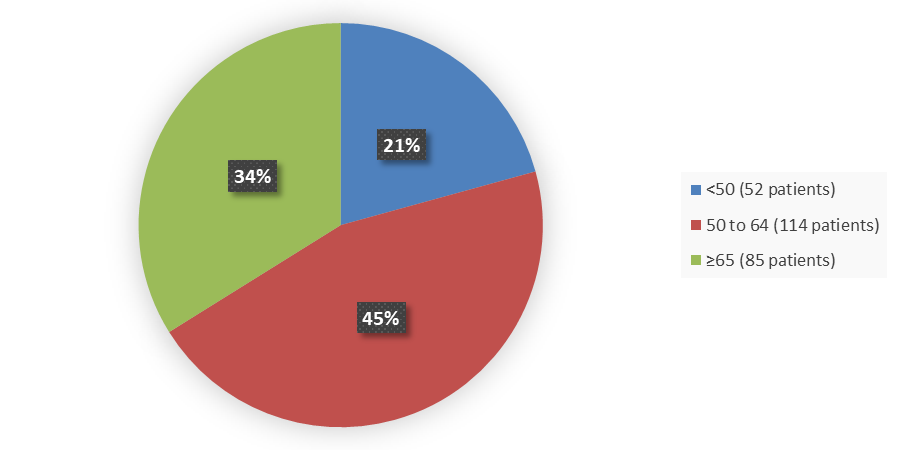
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate the efficacy and side effects of CAMZYOS.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Clinical review
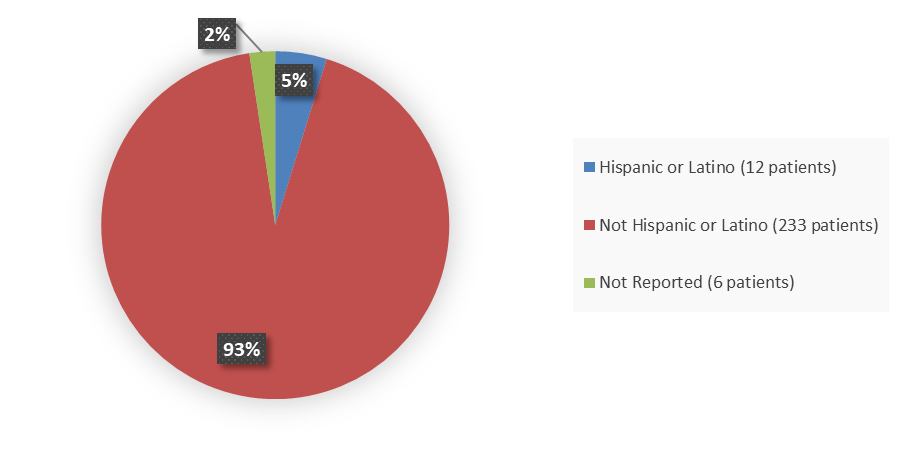
Figure 4 summarizes the percentage of patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy and side effects of CAMZYOS.
Figure 4. Baseline Demographics by Ethnicity
Who participated in the trials?
Table 4 summarize demographics and clinical characteristics of patients in the clinical trial.
Table 4. Demographics and Baseline Characteristics in the Phase 3 Trial
| Demographics and Baseline Characteristics | CAMZYOS N=123 |
Placebo N=128 |
Overall N=251 |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 58.5 (12.2) | 58.5 (11.8) | 58.5 (11.9) |
| Age, n (%) | |||
| ≤49 | 27 (22.0) | 25 (19.5) | 52 (20.7) |
| 50 to 64 | 51 (41.5) | 63 (49.2) | 114 (45.4) |
| ≥65 | 45 (36.6) | 40 (31.3) | 85 (33.9) |
| Sex, n (%) | |||
| Male | 66 (53.7) | 83 (64.8) | 149 (59.4) |
| Female | 57 (46.3) | 45 (35.2) | 102 (40.6) |
| Region, n (%) | |||
| United States | 53 (43.1) | 55 (43.0) | 108 (43.0) |
| Outside the United States | 70 (56.9) | 73 (57.0) | 143 (57.0) |
| Race, n (%) | |||
| White | 115 (93.5) | 114 (89.1) | 229 (91.2) |
| Black or African American | 1 (0.8) | 5 (3.9) | 6 (2.4) |
| American Indian or Alaska Native | 0 | 1 (0.8) | 1 (0.4) |
| Asian | 4 (3.3) | 2 (1.6) | 6 (2.4) |
| Unknown | 3 (2.4) | 6 (4.7) | 9 (3.6) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 8 (6.5) | 4 (3.1) | 12 (4.8) |
| Not Hispanic or Latino | 114 (92.7) | 119 (93.0) | 233 (92.8) |
| Not reported | 1 (0.8) | 5 (3.9) | 6 (2.4) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.7 (4.9) | 29.2 (5.6) | 29.4 (5.3) |
| Range | 20.8, 51.9 | 15.9, 50.1 | 15.9, 51.9 |
| BSA, m2 | |||
| Mean (SD) | 1.96 (0.2) | 1.97 (0.2) | 1.97 (0.2) |
| Range | 1.49, 2.67 | 1.37, 2.74 | 1.37, 2.74 |
| Heart rate, beats/min | |||
| Mean (SD) | 63 (10.1) | 62 (10.6) | 63 (10.3) |
| Blood pressure, mmHg, mean (SD) | |||
| Systolic | 128.4 (16.2) | 128.4 (14.6) | 128.4 (15.4) |
| Diastolic | 75.5 (10.8) | 76.1 (9.9) | 75.8 (10.3) |
| CYP2C19 metabolizer phenotype, n (%) | |||
| Normal | 48 (39.0) | 44 (34.4) | 92 (36.7) |
| Rapid | 27 (22.0) | 32 (25.0) | 59 (23.5) |
| Ultrarapid | 4 (3.3) | 3 (2.3) | 7 (2.8) |
| Intermediate | 31 (25.2) | 33 (25.8) | 64 (25.5) |
| Poor | 2 (1.6) | 3 (2.3) | 5 (2.0) |
| Not poor | 3 (2.4) | 1 (0.8) | 4 (1.6) |
| Missing | 8 (6.5) | 12 (9.4) | 20 (8.0) |
| Duration of oHCM, years | |||
| Mean (SD) | 7 (7.2) | 7 (6.6) | 7.7 (6.9) |
| Range | 0.2, 49.3 | 0.2, 34.4 | 0.2, 49.3 |
| NYHA class, n (%) | |||
| II | 88 (71.5) | 95 (74.2) | 183 (72.9) |
| III | 35 (28.5) | 33 (25.8) | 68 (27.1) |
| Background HCM therapy, n (%) | |||
| Beta-blocker | 94 (76.4) | 95 (74.2) | 189 (75.3) |
| Calcium channel blocker | 25 (20.3) | 17 (13.3) | 42 (16.7) |
| No beta-blocker or calcium channel blocker | 4 (3.3) | 16 (12.5) | 20 (8.0) |
| Baseline NT-proBNP, ng/L | |||
| N | 120 | 126 | 246 |
| Geometric mean (%CV) | 777 (136.4) | 616 (108.4) | 690 (130.8) |
| Mean (SD) | 1516 (2066.4) | 1050 (1138.7) | 1277 (1670.3) |
| Median | 784 | 648 | 710 |
| Q1, Q3 | 373.0, 1759.5 | 354.0, 1360.0 | 366.0, 1610.0 |
| Min; max | 52, 11420 | 18, 6067 | 18, 11420 |
Source: Adapted from FDA Clinical review
Abbreviations: BMI, body mass index; BSA, body surface area; CV, coefficient of variation; HCM, hypertrophic cardiomyopathy; NT proBNP, n-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; oHCM, obstructive hypertrophic cardiomyopathy; Q1, first quartile; Q3, third quartile; SD, standard deviation
How were the trials designed?
The benefits and side effects of CAMZYOS for symptomatic obstructive HCM were evaluated in one clinical trial.
Symptomatic obstructive HCM patients were randomly assigned to receive either CAMZYOS or placebo by mouth once a day. Neither the patients nor the healthcare providers knew which treatment was being given until after the trial was completed.
The benefit of CAMZYOS was evaluated by measuring the percentage of patients who achieved the target improvement in symptoms and the ability to be active after 30 weeks of treatment with CAMZYOS or placebo. Target improvement was a combination of patients’ performance on exercise testing using a bicycle or treadmill and healthcare providers’ assessment on the severity of patient symptoms.
How were the trials designed?
There was one randomized, multicenter, double-blind, placebo-controlled trial that evaluated the safety and efficacy of CAMZYOS.
Enrolled patients were adults with symptomatic NYHA class II and III obstructive HCM, left ventricular ejection fraction (LVEF) ≥55% and Valsalva left ventricular outflow tract (LVOT) peak gradient ≥50 mmHg at rest or with provocation. Patients on dual therapy with beta-blocker and calcium channel blocker treatment, or monotherapy with disopyramide or ranolazine, were excluded.
Patients were randomized in a 1:1 ratio to receive either a starting dose of 5 mg of CAMZYOS or placebo once daily for 30 weeks. The dose was periodically adjusted to optimize patient response (decrease in LVOT gradient with Valsalva maneuver) and maintain LVEF ≥50%
The primary composite functional endpoint, assessed at Week 30, was defined as the proportion of patients who achieved either improvement of peak oxygen consumption (pVO2) by ≥1.5 mL/kg/min plus improvement in NYHA class by at least one or improvement of pVO2 by ≥3.0 mL/kg/min plus no worsening in NYHA class.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.