Drug Trials Snapshots: NEXTSTELLIS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the NEXTSTELLIS Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
NEXTSTELLIS (estetrol and drospirenone)
(NEXT ste LIS)
Mayne Pharmaceuticals, Inc.
Approval date: April 15, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NEXTSTELLIS is a birth control pill (oral contraceptive) used by females to prevent pregnancy.
How is this drug used?
NEXTSTELLIS is taken as one pill every day in the order directed on the blister package.
Who participated in the clinical trials?
The FDA approved NEXTSTELLIS based on evidence from two clinical trials (Study C301/NCT 02817828, Study C302/NCT 02817841) of 3,632 females with reproductive potential who desired a method to prevent pregnancy. The trial was conducted at 146 sites in Europe/Russia (Study C301) and North America (Study C302).
The efficacy population consisted of females from Study C302 (North America) only. The primary safety population consisted of females from Study C302 (North America) with additional supportive safety data from females in Study C301 (Europe/Russia). The reason for this focus is that the demographic composition and baseline characteristics of the population in Study C302 most closely approximates the population in the US likely to use NEXTSTELLIS.
What are the benefits of this drug?
NEXTSTELLIS prevents pregnancy, however, about 2 to 4 out of 100 females may get pregnant during the first year they use NEXTSTELLIS.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary outcome measure was the Pearl Index defined as the number of pregnancies occurring per 100 woman-years (where a woman-year is defined as thirteen 28 day cycles).
A total of 26 on-treatment pregnancies occurred in 1,524 females less than 35 years of age contributing 12,763 at-risk cycles (cycles with at least one episode of heterosexual vaginal intercourse and no back-up contraception use). The overall pregnancy rate, evaluated by the Pearl Index, was 2.65 (95% CI: 1.73, 3.88) per 100 woman-years of NEXTSTELLIS use.
A trend of decreasing effectiveness with increasing BMI was observed in the study.
Table 1. Pearl Index for Females ≤35 Years of Age, by Body Mass Index
| Subgroup | N* | On-Treatment Pregnancies | At-Risk Cycles | Pearl Index (95% CI) |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| <30 | 1187 | 20 | 10113 | 2.57 (1.57, 3.97) |
| ≥30 to <35** | 337 | 6 | 2650 | 2.94 (1.08, 6.41) |
Source: Adapted from NEXTSTELLIS Prescribing Information
* N = all females aged 16 to 35 with at least 1 at-risk cycle.
** One female with a BMI of 48 kg/m2 was enrolled and included in the efficacy analysis.
Abbreviations: BMI, body mass index; CI, confidence interval
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
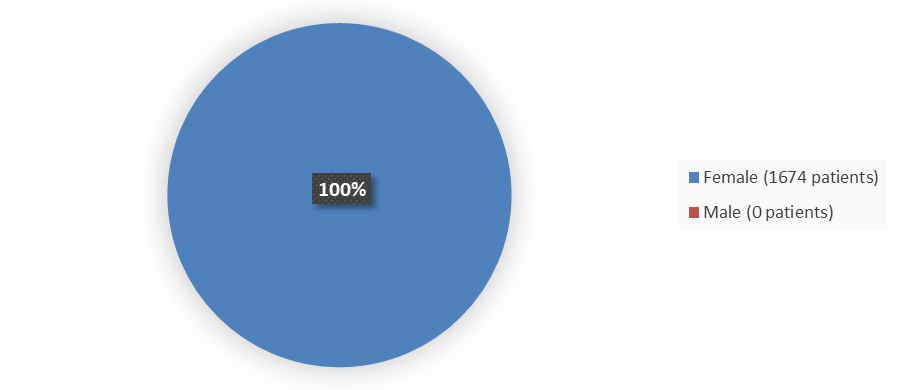
- Sex: All patients in the trials were female.
- Race: NEXTSTELLIS prevents pregnancy in females of all races. There were too few females in some racial groups to draw conclusions about differences in efficacy by racial group.
- Age: All females in the trials were between 16 and 35 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Analysis of efficacy by race shows a numerical difference in the Pearl Index between subgroups as displayed in Table 2. However, there were too few females in some racial groups to draw conclusions. Also, there may be factors other than age and race that may contribute to a difference in the Pearl Index that are not related to the drug’s efficacy.
Table 2. Pearl Index for Females ≤35 Years of Age, by Race
| Subgroup | N | On-Treatment Pregnancies | At-Risk Cycles | Pearl Index (95% CI) |
|---|---|---|---|---|
| Race | ||||
| White | 1090 | 13 | 9570 | 1.77 (0.94, 3.02) |
| Black or African American | 278 | 10 | 1911 | 6.80 (3.26, 12.51) |
| Other | 156 | 3 | 1282 | 3.04 (0.63, 8.89) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval
What are the possible side effects?
Females over 35 years old who smoke should not use NEXTSTELLIS because of serious side effects including death from heart attack, blood clots or stroke. The risk increases with age and the number of cigarettes smoked.
NEXTSTELLIS may cause serious side effects including blood clots, heart attack, stroke, abnormal liver tests, and high blood pressure.
The most common side effects of NEXTSTELLIS are irregular vaginal bleeding (including absence of period), mood changes, headache, breast pain/tenderness/discomfort, painful periods, acne, weight gain, and decreased sex drive.
What are the possible side effects (results of trials used to assess safety)?
Table 3. Adverse Reactions Reported by ≥2% of Females Receiving NEXTSTELLIS
| Preferred Term | Participants With Adverse Reaction | |
|---|---|---|
| US/Canada Phase 3 Trial N=2073* n (%) |
Two Phase 3 Trials N=3632** n (%) |
|
| Any adverse reaction*** | 1205 (58.1) | 2126 (58.5) |
| Mood disturbance1 | 226 (10.9) | 329 (9.1) |
| Bleeding irregularities2 | 201 (9.7) | 393 (10.8) |
| Breast symptoms3 | 110 (5.3) | 197 (5.4) |
| Headache4 | 100 (4.8) | 227 (6.3) |
| Dysmenorrhea5 | 84 (4.1) | 133 (3.7) |
| Weight increased6 | 68 (3.3) | 108 (3.0) |
| Acne7 | 66 (3.2) | 136 (3.7) |
| Libido decreased/lost8 | 27 (1.3) | 72 (2.0) |
Source: Adapted from NEXTSTELLIS Prescribing Information
*Represents the safety population of C302 only (US/Canada).
**Represents the safety population of C301/C302 for DRSP/E4.
***Any adverse reaction equals any adverse event ≥2%.
1 Includes PTs: adjustment disorder, affective disorder, agitation, anger, anxiety, depressed mood, depression, depressive symptom, disorientation, emotional disorder, emotional distress, euphoric mood, generalized anxiety disorder, insomnia, irritability, mood altered, mood swings, nervousness, panic attack, panic disorder, performance fear, restlessness, sleep disorder, stress, suicidal ideation, tearfulness
2 Includes PTs: abnormal withdrawal bleeding, amenorrhea, cervix hemorrhage uterine, coital bleeding, dysfunctional uterine bleeding, menometrorrhagia, menorrhagia, menstrual disorder, menstruation irregular, metrorrhagia, oligomenorrhea, polymenorrhea, uterine hemorrhage, vaginal hemorrhage.
3 Includes PTs: anisomastia, breast cyst, breast discoloration, breast discomfort, breast disorder, breast engorgement, breast enlargement, breast mass, breast edema, breast pain, breast swelling, breast tenderness, fibrocystic breast disease, galactorrhea, gynecomastia, mastoptosis, nipple disorder, nipple pain.
4 Includes PTs headache, premenstrual headache, and tension headache.
5 Includes PTs adnexa uteri pain, dysmenorrhea, premenstrual cramps, pelvic discomfort, pelvic pain, uterine spasm.
6 Includes PTs: weight increased, weight fluctuation, body mass index increased, weight loss poor, and obesity.
7 Includes PTs acne and cystic acne.
8 Includes PTs: libido decreased and loss of libido
Abbreviations: PT, preferred term
Were there any differences in side effects among sex, race and age?
- Sex: All participants were female.
- Race: Differences in the occurrence of side effects in females of different racial backgrounds was not evaluated because there were too few females in some racial groups.
- Age: All females in the trials were between 16 to 50 years of age; therefore, differences in the occurrence of side effects in females below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
No additional information is available on differences in side effects among sex groups as all study participants were females. Trial data was insufficient to assess differences in side effects between racial and age groups because there were too few females in some racial and age groups.
DEMOGRAPHICS SNAPSHOT
Figure 1 summarizes how many males and females were enrolled in the clinical trial used to evaluate the efficacy of NEXTSTELLIS.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
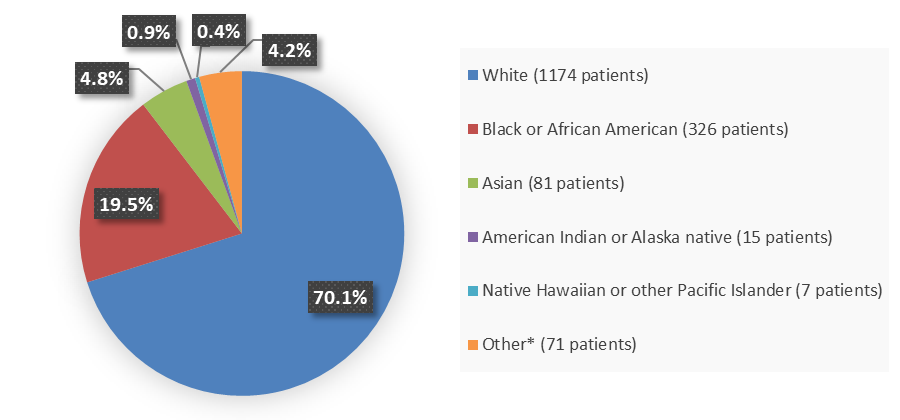
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of NEXTSTELLIS.
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
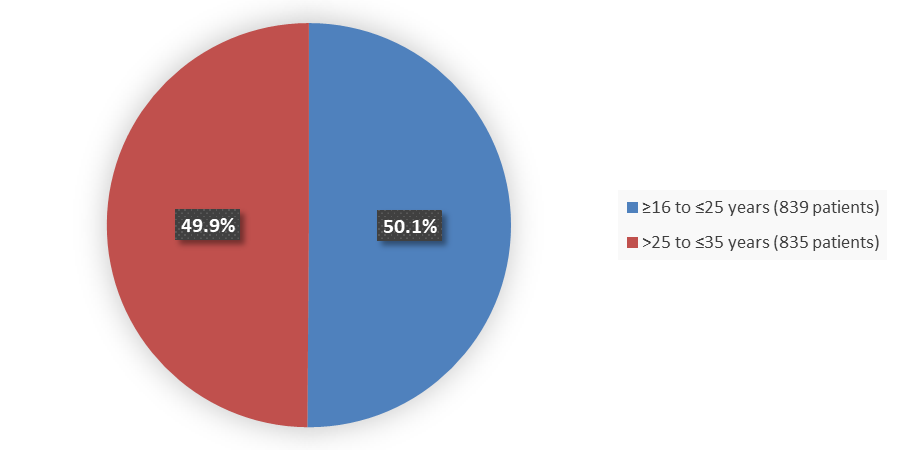
Figure 3. Baseline Demographics by Age Group (Efficacy Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 4. Baseline Demographics
| Demographic | Efficacy Population N=1674 |
|---|---|
| Sex, n (%) | |
| Females | 1674 (100) |
| Race, n (%) | |
| White | 1174 (70) |
| Black or African American | 326 (20) |
| Asian | 81 (5) |
| American Indian or Alaska native | 15 (1) |
| Native Hawaiian or other Pacific Islander | 7 (<1) |
| Other* | 71 (4) |
| Age, years | |
| Median | 25 |
| Min, max | 16, 35 |
| Age group, years, n (%) | |
| ≥16 to ≤25 | 839 (50) |
| >25 to ≤35 | 835 (50) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 429 (26) |
| Not Hispanic or Latino | 1245 (74) |
Source: Adapted from FDA Review
How were the trials designed?
The benefit of NEXTSTELLIS was evaluated in Study C302 (North America), a trial of one-year duration that enrolled females at risk for pregnancy, aged 16 to 35 years, inclusive, and a BMI ≤35.0 kg/m2. The primary efficacy outcome measure was the Pearl Index defined as the number of pregnancies occurring per 100 woman-years (where a woman year is defined as thirteen 28 day menstrual cycles).
The side effects of NEXTSTELLIS were evaluated in two clinical trials: Study C302 (North America) and Study C301 (Europe/Russia). Both trials enrolled females at risk of pregnancy who desired contraception to prevent pregnancy. Study C301 (Europe/Russia) enrolled females aged 18 to 50 years, inclusive, while Study C302 (North America) enrolled females aged 16 to 50 years, inclusive.
How were the trials designed?
The safety and efficacy of NEXTSTELLIS were established in two open-label, single-arm trials: Study C301 (Europe/Russia) and Study C302 (North America). Both trials enrolled sexually active females with regular menstrual cycles. Efficacy was evaluated in females aged 16 to 35 years; safety was evaluated in all females ages 16 to 50 years. All females were to receive NEXTSTELLIS orally once daily in a 24/4-day regimen (i.e., 24 active tablets followed by 4 inactive tablets) for up to a maximum of 13 consecutive cycles.
The efficacy of NEXTSTELLIS was established in Study C302 (North America). The primary endpoint was the Pearl Index defined as the number of pregnancies occurring per 100 woman-years (where a woman year is defined as thirteen 28-day menstrual cycles). The Pearl Index was calculated for females 35 years of age or younger and derived from all cycles in which vaginal intercourse occurred and no back-up contraception was used.
The safety of NEXTSTELLIS was established based primarily on the safety population in Study C302 (North America) with supporting safety information from Study C301 (Europe/Russia). The reason for this focus is that the demographic composition and baseline characteristics of the population in Study C302 most closely approximates the population in the US likely to use this product.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION