Drug Trials Snapshots: VIVJOA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the VIVJOA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
VIVJOA (oteseconazole)
(viv joe' ah)
MYCOVIA PHARMACEUTICALS INC
Approval date: April 26, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VIVJOA is an azole antifungal that is indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are not able to have children.
How is this drug used?
VIVJOA is an oral capsule that can be taken as one of two different dosing regimens as written by your doctor:
A recommended VIVJOA-only dosage regimen in females who are not of reproductive potential is as follows:
- Day 1: Administer VIVJOA 600 mg (as a single dose)
- Day 2: Administer VIVJOA 450 mg (as a single dose)
- Beginning on Day 14: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 2 through 12)
An alternate fluconazole/VIVJOA dosage regimen in females who are not of reproductive potential is as follows:
- Day 1, Day 4, and Day 7: Administer fluconazole 150 mg
- Days 14 through 20: Administer VIVJOA 150 mg once daily for 7 days
- Beginning on Day 28: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 4 through 14)
Who participated in the clinical trials?
The FDA approved VIVJOA based on evidence from 3 clinical trials that enrolled 871 female patients with a history of RVVC (intent-to-treat [ITT] population). The trials were conducted at 157 sites in 11 countries on 3 continents, with most patients from Europe and North America, and several patients were from Asia.
How were the trials designed?
VIVJOA was evaluated in 3 clinical trials of 871 patients in the ITT population with history of RVVC disease.
A total of 656 adults and post-menarchal pediatric females with RVVC (defined as ≥3 episodes of vulvovaginal candidiasis [VVC] in a 12-month period) were randomized in two multicenter, multinational, double-blind, placebo-controlled trials: Trial 1 (NCT#03562156) and Trial 2 (NCT#03561701). A total of 219 adults and post-menarchal pediatric females with RVVC were randomized in a multicenter, double-blind trial, Trial 3 (NCT#03840616).
In Trials 1 and 2, patients with a history of RVVC who had an active repeat VVC infection and who were successfully treated with a few days of fluconazole (2-week induction phase) were then randomized to receive VIVJOA or placebo for 12 weeks (maintenance phase). Neither the patients nor the health care providers knew who was being given which treatment until after the trial was completed.
Trial 3 was a randomized clinical trial studying patients with a history of RVVC who received VIVJOA versus fluconazole in the induction phase (2 weeks) and if successfully treated then received VIVJOA versus placebo for 11 weeks during the maintenance phase. Neither the patients nor the health care providers knew who was being given which treatment until after the trial was completed.
Patients in Trials 1 and 2 were followed up for 36 weeks after 12 weeks of maintenance therapy. Patients in Trial 3 were followed up for 37 weeks after maintenance therapy.
The benefit of VIVJOA was measured by the proportion of patients with one of more culture verified acute VVC episodes during the maintenance phase through Week 48 for Trials 1 and 2 and by the proportion of patients with one or more culture verified acute VVC episode during the maintenance phase through Week 50 or who failed clearing their infection during the induction phase.
How were the trials designed?
FDA approved VIVJOA based on data from three randomized, multi-center, controlled, parallel-arm, double-blind, superiority trials. Two trials were placebo-controlled, and one trial was active/placebo controlled.
Trials 1 and 2 enrolled female patients with RVVC (defined as ≥3 episodes of VVC in a 12 month period). Both trials consisted of two phases: an open-label induction phase and an 11-week maintenance phase. Patients received three sequential doses of 150 mg of fluconazole (every 72 hours) on Days 1, 4, and 7 during the induction phase. Patients returned 14 days after the first dose of fluconazole and if the acute VVC episode was resolved (vulvovaginal signs and symptoms score <3) they were randomized (2:1) to receive either 150 mg of VIVJOA or placebo for 7 days followed by 11 weekly doses in the maintenance phase.
The primary endpoint in Trials 1 and 2 was the proportion of patients with ≥1 culture verified acute VVC episode (positive fungal culture for Candida species associated with a clinical signs and symptoms score of ≥3) during the maintenance phase through Week 48.
Trial 3 enrolled female patients with RVVC. The trial consisted of two phases: induction and maintenance. Patients were randomized 2:1 to receive VIVJOA or fluconazole/placebo. During the induction phase, patients received 1050 mg of VIVJOA over two days (600 mg on Day 1 and 450 mg on Day 2) or three sequential doses of 150 mg of fluconazole (every 72 hours) on Days 1, 4, and 7. Patients returned 14 days after the first dose and moved to the maintenance phase if the acute VVC episode was resolved. During the maintenance phase, patients received 150 mg VIVJOA weekly or placebo weekly for 11 weeks.
The primary endpoint in Trial 3 was the proportion of patients with ≥1 culture verified acute VVC episode during the maintenance phase through Week 50 or who failed clearing their infection during the induction phase.
DEMOGRAPHICS SNAPSHOT
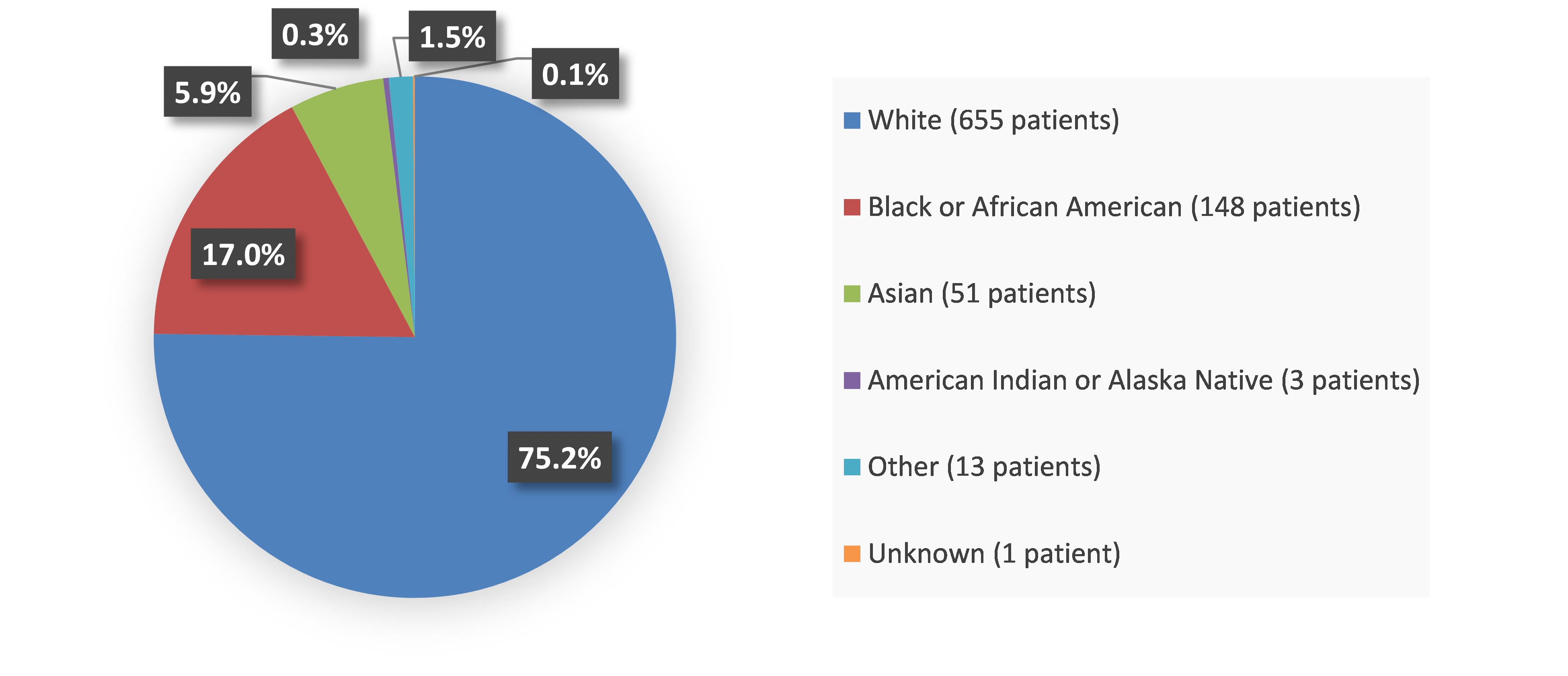
Figure 1. Baseline Demographics by Race
Source: Adapted from FDA Review
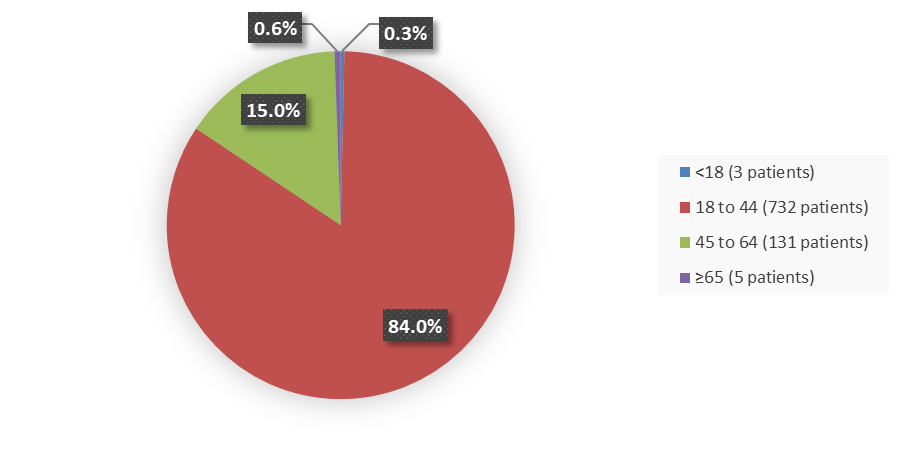
Figure 2. Baseline Demographics by Age
Source: Adapted from FDA Review
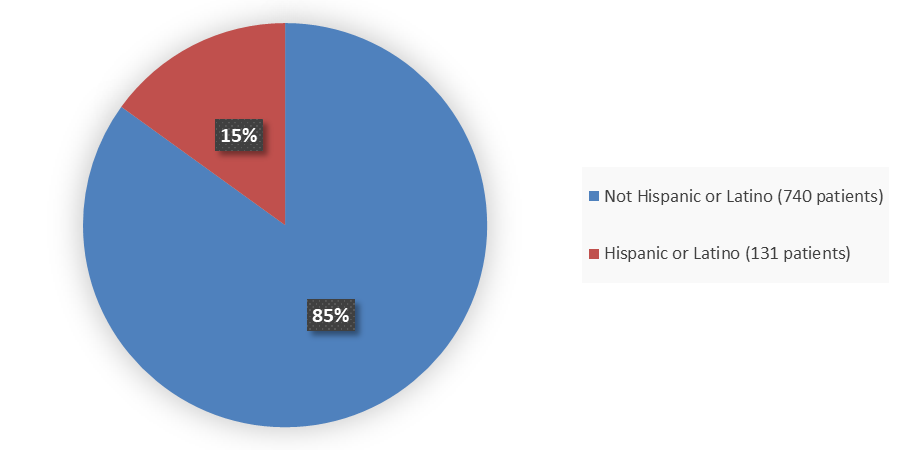
Figure 3. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
The following table summarizes the demographics for the ITT population (all randomized subjects) in the clinical trials.
Table 1. Demographics Trials 1, 2, and 3 (ITT Population)
| Parameter | Trial 1 | Trial 2 | Trial 3 | |||
|---|---|---|---|---|---|---|
| VIVJOA N=217 |
Placebo N=109 |
VIVJOA N=218 |
Placebo N=108 |
VIVJOA N=147 |
Fluconazole/ Placebo N=72 |
|
| Age, years | ||||||
| Mean (SD) | 34 (10.3) | 34 (9.9) | 34 (9.4) | 36 (10.8) | 34 (10.7) | 36 (11.7) |
| Median (min, max) | 33 (17, 78) | 34 (14, 16) | 33 (18, 61) | 34 (18, 73) | 34 (16, 66) | 36 (16, 78) |
| Age group, years, n (%) | ||||||
| 12 to 17 | 1 (<1) | 0 | 0 | 0 | 1 (<1) | 1 (1) |
| 18 to 33 | 109 (50) | 54 (50) | 111 (51) | 54 (50) | 72 (49) | 30 (42) |
| ≥34 | 107 (49) | 55 (50) | 107 (49) | 54 (50) | 74 (50) | 41 (57) |
| Race, n (%) | ||||||
| White | 156 (72) | 80 (73) | 193 (89) | 96 (89) | 88 (60) | 42 (58) |
| Black or African American | 26 (12) | 17 (16) | 23 (11) | 8 (7) | 47 (32) | 27 (38) |
| Asian | 33 (15) | 12 (11) | 0 | 0 | 0 | 0 |
| American Indian or Alaska Native | 1 (<1) | 0 | 0 | 0 | 0 | 0 |
| Other | 1 (<1) | 0 | 2 (<1) | 4 (4) | 12 (8) | 3 (4) |
| Ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 198 (91) | 103 (94) | 187 (96) | 90 (83) | 109 (74) | 162 (74) |
| Hispanic or Latino | 19 (9) | 6 (6) | 30 (14) | 18 (17) | 38 (26) | 57 (26) |
Source: Adapted from FDA review
What are the benefits of this drug?
VIVJOA worked better than the placebo comparator in lowering the likelihood of RVVC infections.
What are the benefits of this drug (results of trials used to assess efficacy)?
VIVJOA was superior to placebo with reference to the proportion of patients with ≥1 culture‑verified acute VVC episode through Week 48 (Table 1) or the proportion of patients with ≥1 culture verified acute VVC episode or who took medication known to treat VVC during the maintenance phase through Week 48. For both Trial 1 and Trial 2, the average percentage of patients was lower in the VIVJOA groups compared with the placebo group (Table ).
Table 2. Trial 1 and 2 Efficacy Endpoints: ITT Population
| Parameter | Trial 1 | Trial 2 | ||
|---|---|---|---|---|
| VIVJOA N=217 % |
Placebo N=109 % |
VIVJOA N=218 % |
Placebo N=108 % |
|
| Proportion of patients with ≥1 culture‑verified acute VVC episode (Day 1 through Week 48)a | 6.7 | 42.8 | 3.9 | 39.4 |
| Treatment difference p-valueb | <0.001 | <0.001 | ||
| Proportion of patients with ≥1 culture‑verified acute VVC episode or received VVC medication (Day 1 through Week 48)a | 27.3 | 50.8 | 21.3 | 49.7 |
| Treatment difference p-valueb | <0.001 | <0.001 | ||
Source: VIVJOA Prescribing information
a. Average %. Missing values were imputed with multiple imputation using the following auxiliary information: region, treatment, Baseline body mass index, Baseline age, ethnicity, and visit.
b. The p-value was obtained using a Chi-square test comparing VIVJOA with placebo.
Abbreviations: ITT, intent-to-treat population; VVC, vulvovaginal candidiasis
VIVJOA was superior to fluconazole/placebo in the proportion of patients with ≥1 culture‑verified recurring acute VVC episode during the maintenance phase (post randomization through Week 50) or failed clearing their infection during the induction phase and the proportion of patients with ≥1 culture‑verified recurring acute VVC episode or took VVC medication known to treat VVC during the maintenance phase (post randomization through Week 50) or who failed clearing their infection during the induction phase. The average percentage of patients was lower in the VIVJOA group compared with the fluconazole/placebo group (Table ).
Table 3. Trial 3 Efficacy Endpoints ITT population
| Parameter | VIVJOA N=147 % |
Fluconazole/ Placebo N=72 % |
Treatment Difference p-valueb |
|---|---|---|---|
| Proportion of patients with ≥1 culture-verified acute VVC episode through Week 50 or unresolved VVC episode during the induction phasea | 10.3 | 42.9 | <0.001 |
| Proportion of patients with ≥1 culture-verified acute VVC episode or took VVC medication through Week 50 or unresolved VVC episode during the induction phasea | 43.5 | 59.0 | 0.039 |
Source: VIVJOA Prescribing Information
a. Average %, Missing values were imputed with multiple imputation using the following auxiliary information: treatment, baseline body mass index, baseline age, ethnicity, and visit.
b. The p-value was obtained using a Chi-square test comparing VIVJOA with fluconazole/placebo.
Abbreviations: ITT, intent-to-treat population; VVC, vulvovaginal candidiasis
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: VIVJOA is only for females who are not of reproductive potential. Males were not included in any of the clinical trials.
- Race: VIVJOA worked similarly in White and Black/African American patients.
- Age: VIVJOA worked similarly in patients younger and older than 34 years of age. There were too few persons 65 years of age and older to determine any differences in efficacy between patients older than 65 years of age and those patients younger than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
For Trial 1, the proportion of subjects with ≥1 culture verified acute VVC episode during the maintenance phase by age group and race were significantly lower in VIVJOA as compared to placebo.
Table 4. Subgroup Analyses of Primary Endpoint CL-011: ITT Population
| Subgroup | VIVJOA N=217 |
Placebo N=109 |
P-value |
|---|---|---|---|
| Age group, years | |||
| 18 to 33 | N=109 | N=54 | <0.001 |
| Average % (MI)a | 4.9 | 49.3 | <0.001 |
| Number recurrence (%)b | 27 (24.8) | 34 (63.0) | <0.001 |
| ≥34 | N=107 | N=55 | |
| Average % (MI) | 7.7 | 36.5 | <0.001 |
| Number recurrence (%) | 40 (25.6) | 40 (50.0) | <0.001 |
| Race | |||
| White | N=156 | N=80 | |
| Average % (MI)a | 6.0 | 39.1 | <0.001 |
| Number recurrence (%)b | 40 (25.6) | 40 (50.0) | <0.001 |
| Black or African American | N=26 | N=17 | |
| Average % (MI)a | 19.6 | 47.1 | 0.054 |
| Number recurrence (%)b | 13 (50.0) | 8 (47.1) | <0.001 |
| Other | N=35 | N=12 | |
| Average % (MI)a | 0.3 | 61.7 | <0.001 |
| Number recurrence (%)b | 3 (8.6) | 8 (66.7) | <0.001 |
Source: Adapted from FDA Review
a. Missing values were imputed with multiple imputation (MI) using the following auxiliary information: region, treatment, baseline body mass index, baseline age, ethnicity, and visit.
b. Subjects with incomplete follow-up had their missing values imputed as failures.
The p-value was obtained using a Chi-square test comparing active treatment to placebo.
For Trial 2, the proportion of subjects with ≥1 culture verified acute VVC episode during the maintenance phase by age group and race were significantly lower in VIVJOA as compared to placebo.
Table 5. Subgroup Analyses of Primary Endpoint CL-012: ITT Population
| Subgroup | VIVJOA N=218 |
Placebo N=108 |
P-value |
|---|---|---|---|
| Age group, years | |||
| 18 to 33 | N=111 | N=54 | <0.001 |
| Average % (MI)a | 5.6 | 35.7 | <0.001 |
| Number recurrence (%)b | 26 (23.4) | 24 (44.4) | 0.006 |
| ≥34 | N=107 | N=54 | |
| Average % (MI) | 2.1 | 43.1 | <0.001 |
| Number recurrence (%) | 20 (18.7) | 28 (51.9) | <0.001 |
| Race | |||
| White | N=193 | N=96 | |
| Average % (MI)a | 3.7 | 37.2 | <0.001 |
| Number recurrence (%)b | 38 (19.7) | 43 (44.8) | <0.001 |
| Black or African American | N=23 | N=8 | |
| Average % (MI)a | 5.7 | 48.8 | 0.054 |
| Number recurrence (%)b | 8 (34.8) | 6 (75.0) | 0.049 |
| Other | N=2 | N=4 | |
| Average % (MI)a | 0.0 | 75.0 | NA |
| Number recurrence (%)b | 0 | 3 (75.0) | NA |
Source: Adapted from FDA Review
a. Missing values were imputed with MI using the following auxiliary information: region, treatment, baseline body mass index, baseline age, ethnicity, and visit.
b. Missing were imputed as failures
The p-value was obtained using a Chi-square test comparing active treatment to placebo.
Abbreviations: NA, not applicable
For Trial 3, the proportion of subjects with ≥1 culture verified acute VVC episode during the post-randomization through Week 50 by age group and race (except Other) were significantly lower in VIVJOA as compared to placebo.
Table 6. Subgroup Analyses of Primary Endpoint CL-017: ITT Population
| Subgroup | VIVJOA N=147 |
Fluconazole/Placebo N=72 |
P-value |
|---|---|---|---|
| Age group, years | |||
| 18 to 33 | N=72 | N=30 | |
| Average % (MI)a | 7.2 | 47.3 | <0.001 |
| Number recurrence (%)b | 20 (27.8) | 18 (60.0) | 0.002 |
| ≥34 | N=74 | N=41 | |
| Average % (MI) | 13.4 | 40.7 | 0.002 |
| Number recurrence (%) | 24 (32.4) | 22 (53.7) | 0.026 |
| Race | |||
| White | N=88 | N=42 | |
| Average % (MI)a | 6.3 | 41.2 | <0.001 |
| Number recurrence (%)b | 26 (29.6) | 21 (50.0) | 0.023 |
| Black or African American | N=47 | N=27 | |
| Average % (MI)a | 18.3 | 50.4 | <0.001 |
| Number recurrence (%)b | 16 (34.0) | 19 (70.4) | 0.003 |
| Other | N=12 | N=3 | |
| Average % (MI)a | 8.3 | 0 | NE |
| Number recurrence (%)b | 2 (16.7) | 0 | 0.448 |
Source: Adapted from FDA Review
a. Missing values were imputed with multiple imputation (MI) using the following auxiliary information: region, treatment, baseline body mass index, baseline age, ethnicity, and visit.
b. Subjects with incomplete follow-up had their missing values imputed as failures.
The p-value was obtained using a Chi-square test comparing active treatment to placebo.
Abbreviations: N, number of patients in treatment arm; n, number of patients with adverse event
What are the possible side effects?
VIVJOA may cause serious side effects including allergic reactions. VIVJOA may cause harm to an unborn fetus and is not recommended for use in people who are pregnant or breastfeeding.
The most common side effects in patients treated with VIVJOA in the clinical trials were headache and nausea.
Short-lived increases in creatine phosphokinase (a test for muscle injury) were seen more often in patients treated with VIVJOA than in the comparator in the clinical trials.
What are the possible side effects (results of trials used to assess safety)?
Table 6. Patients With Common Adverse Events Occurring at ≥2% Frequency in Trials VMT-VT-1161-CL-017, VMT-VT-1161-CL-011, and VMT-VT-1161-CL-012, Safety Population
| Preferred Term | Oteseconazole 1050mg/150mg 11 Weeks N=580 n (%) |
Placebo N=291 n (%) |
Total N=871 n (%) |
|
|---|---|---|---|---|
| Any AE | 320 (55.2) | 172 (59.1) | 492 (56.5) | |
| Bacterial vaginosis | 44 (7.6) | 28 (9.6) | 72 (8.3) | |
| Urinary tract infection | 42 (7.2) | 23 (7.9) | 65 (7.5) | |
| Nasopharyngitis | 40 (6.9) | 15 (5.2) | 55 (6.3) | |
| Headache | 34 (5.9) | 21 (7.2) | 55 (6.3) | |
| Nausea | 21 (3.6) | 7 (2.4) | 28 (3.2) | |
| Sinusitis | 19 (3.3) | 8 (2.7) | 27 (3.1) | |
| Upper respiratory tract infection | 18 (3.1) | 16 (5.5) | 34 (3.9) | |
| Influenza | 16 (2.8) | 7 (2.4) | 23 (2.6) | |
| Pyrexia | 13 (2.2) | 8 (2.7) | 21 (2.4) | |
| Diarrhoea | 12 (2.1) | 7 (2.4) | 19 (2.2) | |
| Vulvovaginal pruritus | 12 (2.1) | 11 (3.8) | 23 (2.6) | |
| Back pain | 10 (1.7) | 9 (3.1) | 19 (2.2) | |
| Cystitis | 9 (1.6) | 12 (4.1) | 21 (2.4) | |
| Abdominal pain | 7 (1.2) | 6 (2.1) | 13 (1.5) | |
| Vaginal discharge | 3 (0.5) | 6 (2.1) | 9 (1.0) | |
| Vulvovaginal candidiasis | 3 (0.5) | 6 (2.1) | 9 (1.0) | |
Source: adae.xpt (eCTD seq 0038); FDA reviewer's analysis
Treatment-emergent adverse events defined as any event reported on the eCRF that occurred on or after the initiation of study drug. AEs occurring on Day 1 with no associated onset time are assumed to be treatment emergent.
Duration is approx. 50 weeks.
Coded as MedDRA preferred terms.
Abbreviati
Were there any differences in side effects among sex, race and age?
- Sex: VIVJOA is only to be used by females. The most common side effects were headache and nausea.
- Race: Most of the patients in the clinical trials were White. Differences in the occurrence of headache and nausea among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of headache and nausea was similar in patients younger and older than 45 years of age. There were too few persons 65 years of age and older to determine any differences in safety between patients older than 65 years of age and those patients younger than 65 years of age.
- Ethnicity: The occurrence of headache and nausea side effects was similar in Hispanic or Latino and not Hispanic or Latino patients.
There were no major differences in adverse reaction by race, age groups, or body mass index.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 7. Overview of Side Effects by Sex, Race, and Age in Trials VMT-VT-1161-CL-017, VMT-VT-1161-CL-011, and VMT-VT-1161-CL-012, Safety Population
| Oteseconazole 1050mg/150mg 11 Weeks N=580 |
Placebo N=291 |
Total N=871 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
All Patients n (%) |
All Grades n/Ns (%) |
Grades 3 to 4 n/Ns (%) |
| Sex, n (%) | |||||||||
| Female | 580 (100) | 320/580 (55.2) | 22/580 (3.8) | 291 (100) | 172/291 (59.1) | 8/291 (2.7) | 871 (100) | 492/871 (56.5) | 30/871 (3.4) |
| Race, n (%) | |||||||||
| White | 435 (75.0) | 220/435 (50.6) | 17/435 (3.9) | 219 (75.3) | 124/219 (56.6) | 5/219 (2.3) | 654 (75.1) | 344/654 (52.6) | 22/654 (3.4) |
| Black or African American | 96 (16.6) | 61/96 (63.5) | 3/96 (3.1) | 53 (18.2) | 33/53 (62.3) | 2/53 (3.8) | 149 (17.1) | 94/149 (63.1) | 5/149 (3.4) |
| Asian | 36 (6.2) | 33/36 (91.7) | 0/36 (0) | 15 (5.2) | 13/15 (86.7) | 1/15 (6.7) | 51 (5.9) | 46/51 (90.2) | 1/51 (2.0) |
| American Indian or Alaska Native | 2 (0.3) | 1/2 (50.0) | 1/2 (50.0) | 1 (0.3) | 1/1 (100) | 0/1 (0) | 3 (0.3) | 2/3 (66.7) | 1/3 (33.3) |
| Other | 10 (1.7) | 4/10 (40.0) | 1/10 (10.0) | 3 (1.0) | 1/3 (33.3) | 0/3 (0) | 13 (1.5) | 5/13 (38.5) | 1/13 (7.7) |
| Unknown | 1 (0.2) | 1/1 (100) | 0/1 (0) | 0 (0) | 0/0 (NA) | 0/0 (NA) | 1 (0.1) | 1/1 (100) | 0/1 (0) |
| Age group, years, n (%) | |||||||||
| <18 | 2 (0.3) | 0/2 (0) | 0/2 (0) | 1 (0.3) | 1/1 (100) | 0/1 (0) | 3 (0.3) | 1/3 (33.3) | 0/3 (0) |
| 18-44 | 487 (84.0) | 267/487 (54.8) | 15/487 (3.1) | 241 (82.8) | 141/241 (58.5) | 7/241 (2.9) | 728 (83.6) | 408/728 (56.0) | 22/728 (3.0) |
| 45-65 | 88 (15.2) | 51/88 (58.0) | 6/88 (6.8) | 47 (16.2) | 28/47 (59.6) | 1/47 (2.1) | 135 (15.5) | 79/135 (58.5) | 7/135 (5.2) |
| >65 | 3 (0.5) | 2/3 (66.7) | 1/3 (33.3) | 2 (0.7) | 2/2 (100) | 0/2 (0) | 5 (0.6) | 4/5 (80.0) | 1/5 (20.0) |
Source: adae.xpt (eCTD seq 0038); FDA reviewer's analysis
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; Ns, total number of patients in each category
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.