Drug Trials Snapshots: VTAMA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the VTAMA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
VTAMA (tapinarof)
(vee-TAM-uh)
Dermavant Sciences Inc.
Approval date: May 23, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VTAMA is a small molecule aryl hydrocarbon receptor (AhR) modulating agent that is indicated for the topical treatment of plaque psoriasis in patients 18 years of age and older.
How is this drug used?
VTAMA is a topical cream that is applied to affected areas once daily.
Who participated in the clinical trials?
The FDA approved VTAMA based on evidence (efficacy and safety) from two clinical trials of 1,025 patients with plaque psoriasis. The trials were conducted at 97 sites in the United States and Canada.
How were the trials designed?
The efficacy and safety of VTAMA were evaluated in two clinical trials (DMVT-505-3001 and DMVT-505-3002) of adult patients with stable plaque psoriasis. Both trials were multicenter, randomized, double-blind, vehicle-controlled trials. In both trials, patients applied the drug once daily to affected areas for 12 weeks. The benefit of VTAMA to vehicle was assessed after 12 weeks of treatment using the Physician’s Global Assessment (PGA) score that measures the severity of disease on a scale from 0 to 4 for both trials.
Because plaque psoriasis is a chronic disease, long-term data is necessary to assess the safety and efficacy. After completing the initial 12-week daily phase, patients could voluntarily continue in an open-label, long-term extension study (DMVT-505-3003) for up to an additional 40 weeks.
How were the trials designed?
The efficacy and safety of VTAMA were evaluated in two clinical trials (DMVT-505-3001 and DMVT-505-3002) of adult patients with plaque psoriasis. Both trials were multicenter, randomized, double-blind, vehicle-controlled trials for 12 weeks. All patients had stable plaque psoriasis for at least 6 months, a body surface area involvement between 3% to 20%, and at least a PGA score ≥2 (mild).
In both trials, patients applied the drug once daily to affected areas. The benefit of VTAMA compared to the vehicle was assessed after 12 weeks of treatment using the static PGA score that measures the severity of disease on a scale from 0 to 4 for both trials. The primary efficacy outcome measure for both trials was the proportion of patients at Week 12 who achieved success, defined as a PGA score of 0 (clear) or 1 (almost clear) with a 2-grade or greater improvement from baseline, comparing VTAMA-treated patients to vehicle-treated patients.
After completing the initial 12-week phase, patients could voluntarily continue in an open-label, long-term extension study (DMVT-505-3003) for up to an additional 40 weeks. In this phase, VTAMA was only applied as needed. Patients entering DMVT-505-3003 with a PGA ≥1 (almost clear or worse) were treated with VTAMA until they achieved complete disease clearance (PGA score of 0 ), at which time treatment was stopped. If or when the subject had a flare, worsening of disease (PGA ≥2 [mild or worse]), the subject would reinitiate treatment until a PGA score of 0 (clear) was achieved.
DEMOGRAPHICS SNAPSHOT
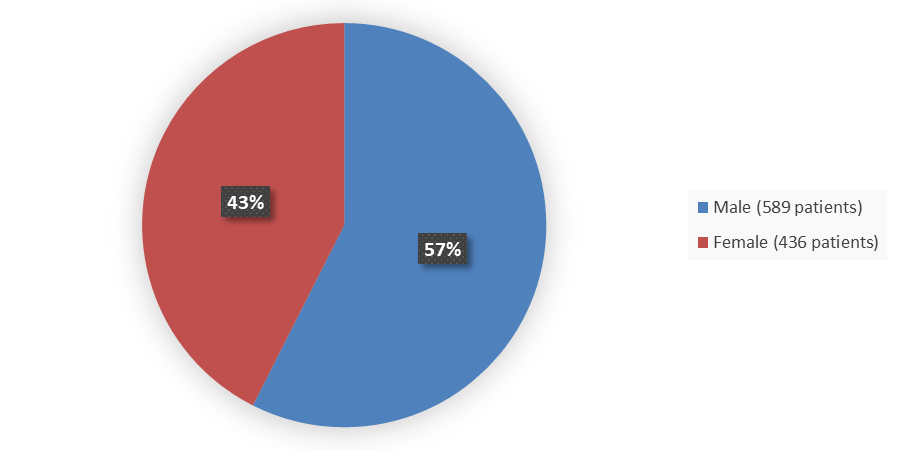
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
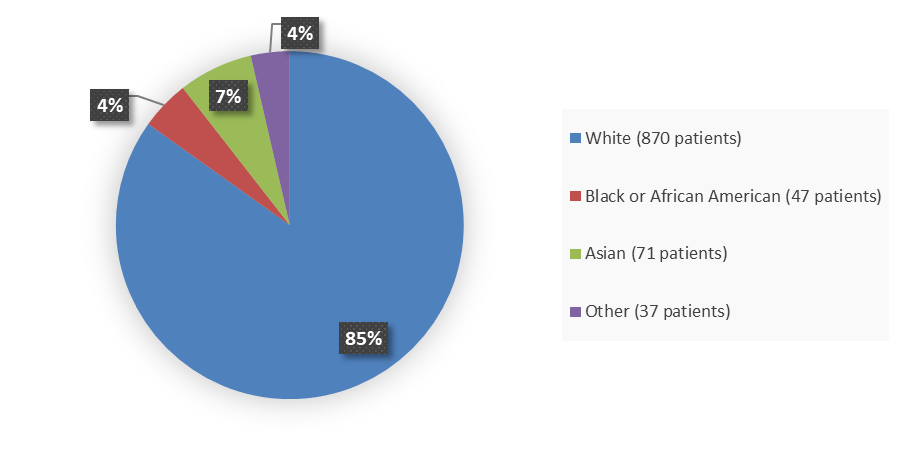
Figure 2. Baseline Demographics by Race
Adapted from FDA Review
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
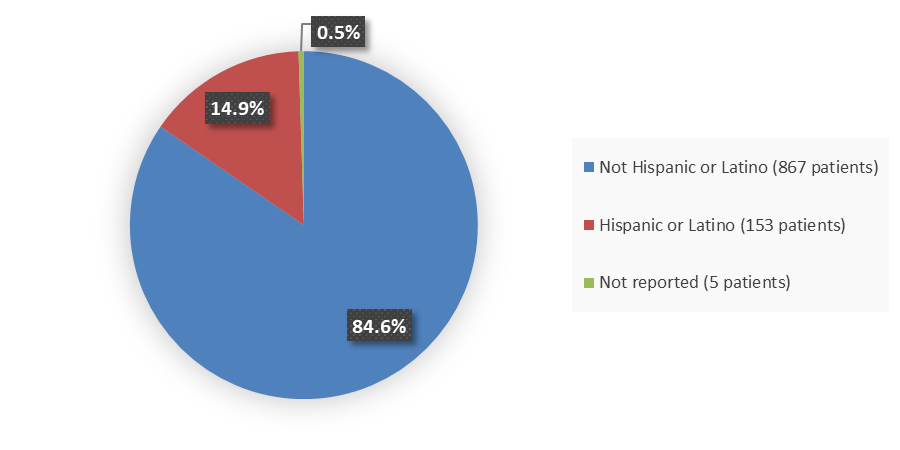
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes demographics for the randomized patients in the clinical trials, DMVT-505-3001 and DMVT-505-3002.
Table 1. Demographics and Baseline Disease Characteristics (ITT1)
| Demographic | Trial DMVT-505-3001 | Trial DMVT-505-3002 | ||
|---|---|---|---|---|
| VTAMA N=340 |
Vehicle N=170 |
VTAMA N=343 |
Vehicle N=172 |
|
| Age, years | ||||
| Mean (SD) | 50 (14) | 49 (13) | 50 (13) | 50 (14) |
| Median | 51 | 51 | 51 | 52 |
| Min, max | 18, 75 | 21, 74 | 19, 75 | 18, 74 |
| Age group, years, n (%) | ||||
| <65 | 286 (84) | 158 (93) | 298 (87) | 144 (84) |
| ≥65 | 54 (16) | 12 (7) | 45 (13) | 28 (16) |
| Sex, n (%) | ||||
| Male | 213 (63) | 86 (51) | 188 (55) | 102 (59) |
| Female | 127 (37) | 84 (49) | 155 (45) | 70 (41) |
| Race, n (%) | ||||
| White | 286 (84) | 146 (86) | 300 (87) | 138 (80) |
| Black or African American | 18 (5) | 11 (6) | 12 (3) | 6 (3) |
| Asian | 21 (6) | 4 (2) | 25 (7) | 21 (12) |
| Other | 15 (4) | 9 (5) | 6 (2) | 7 (4) |
| Ethnicity, n (%) | ||||
| Hispanic | 53 (16) | 34 (20) | 43 (13) | 23 (13) |
| Not Hispanic | 284 (84) | 135 (79) | 300 (87) | 148 (86) |
| Not reported | 3 (1) | 1 (1) | 0 | 1 (1) |
| Country, n (%) | ||||
| United States | 248 (73) | 129 (76) | 266 (78) | 133 (77) |
| Canada | 92 (27) | 41 (24) | 77 (22) | 39 (23) |
| PGA, n (%) | ||||
| 2 – mild | 39 (11) | 21 (12) | 28 (8) | 15 (9) |
| 3 – moderate | 271 (80) | 133 (78) | 288 (84) | 144 (84) |
| 4 – severe | 30 (9) | 16 (9) | 27 (8) | 13 (8) |
| PASI | ||||
| Mean (SD) | 8.7 (4.0) | 9.2 (4.4) | 9.1 (3.7) | 9.3 (4.0) |
| Median | 8.1 | 8.0 | 8.4 | 8.8 |
| Min, max | 2.0, 22.2 | 2.0, 21.9 | 1.5, 23.6 | 2.5, 25.0 |
| Percent BSA | ||||
| Mean (SD) | 7.8 (4.6) | 8.2 (5.1) | 7.8 (4.4) | 7.3 (4.1) |
| Median | 6.3 | 6.1 | 6.2 | 6.0 |
| Min, max | 3.0, 20.0 | 3.0, 20.0 | 3.0, 20.0 | 3.0, 20.0 |
Source: Adapted from FDA Review
1 ITT population: all randomized subjects.
Abbreviations: BSA, body surface area; ITT, intent-to-treat; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; SD, standard deviation
What are the benefits of this drug?
In two trials, more patients with plaque psoriasis achieved clear or almost clear skin after 12 weeks of treatment with VTAMA in comparison to those who were treated with placebo. In Trial DMVT-505-3001, 35% of patients treated with VTAMA achieved clear or almost clear skin compared to 6% of patients treated with placebo. In Trial DMVT-505-3002, 40% of patients treated with VTAMA achieved clear or almost clear skin compared to 6% of patients treated with placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 comparing VTAMA-treated patients to placebo-treated patients summarizes efficacy results for the evaluated patients in Trials DMVT-505-3001 and DMVT-505-3002. The primary efficacy endpoint was the proportion of subjects who achieved a PGA score of 0 (clear) or 1 (almost clear) with a ≥2-grade improvement from baseline at Week 12.
Table 2. Efficacy Results in Adults With Psoriasis (ITT1) in DMVT-505-3001 and DMVT-505-3002
| Trial DMVT-505-3001 | Trial DMVT-505-3002 | |||
| PGA Response2 at Week 12 | VTAMA N=340 |
Placebo N=170 |
VTAMA N=343 |
Placebo N=172 |
| Proportion3, (%) | 35 | 6 | 40 | 6 |
| % Difference (95% CI)3 | 29 (23, 36) | 34 (27, 41) | ||
| Relative risk (95% CI)4 | 5.8 (2.9, 11.5) | 6.1 (3.3, 11.4) | ||
| P-value4 | <0.001 | <0.001 | ||
Source: Adapted from FDA Review
1 ITT population: all randomized subjects. Missing data were imputed using multiple imputation.
2 Response is defined as a PGA score of 0 (clear) or 1 (almost clear) with a ≥2-grade improvement from baseline.
3 Average over the 100 imputed datasets. The standard error for calculating the 95% CI was based on Rubin’s rule (i.e., reflects both within and across the 100 imputed datasets).
4 Relative risk, 95% CI, and p-value are based on a CMH test stratified by baseline PGA score (i.e., the factor used to stratify the randomization).
Abbreviations: CI, confidence interval; CMH, Cochran-Mantel-Haenszel; ITT, intent-to-treat; PGA, Physician’s Global Assessment
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: VTAMA worked similarly in males and females.
- Race: The number of patients of races other than White was limited; therefore, differences in response among races could not be determined.
- Age: The number of patients older than 65 years of age was limited; therefore, differences in response between patients younger and older than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 and Table 4 summarize efficacy results at Week 12 by age, sex, and race.
Table 3. PGA Response1 at Week 12 by Age, Sex, Race, Ethnicity, Country, and Baseline PGA Score (ITT2) for Trial DMVT-505-3001
| Subgroup (n[VTAMA], n[Vehicle]) |
VTAMA N=340 % |
Vehicle N=170 % |
Difference % (95% CI) |
| Age, years | |||
| <65 (286, 158) | 34 | 6 | 27 (20, 35) |
| ≥65 (54, 12) | 46 | 9 | 37 (16, 59) |
| Sex | |||
| Male (213, 86) | 36 | 6 | 30 (21, 39) |
| Female (127, 84) | 35 | 7 | 28 (17, 39) |
| Race | |||
| White (286, 146) | 36 | 7 | 29 (22, 37) |
| Black (18, 11) | 20 | 1 | 19 (-3, 40) |
| Asian (21, 4) | 48 | 5 | 43 (7, 79) |
| Other (15, 9) | 25 | 3 | 22 (-6, 50) |
| Ethnicity | |||
| Hispanic (53, 34) | 30 | 2 | 28 (13, 42) |
| Not Hispanic (284, 135) | 37 | 7 | 29 (21, 37) |
| Not reported (3, 1) | 33 | 0 | - |
| Country | |||
| United States (248, 129) | 33 | 5 | 28 (21, 36) |
| Canada (92, 41) | 41 | 10 | 31 (17, 46) |
| Baseline PGA score | |||
| 2 – mild (39, 21) | 17 | 5 | 12 (-3, 28) |
| 3 – moderate (271, 133) | 37 | 6 | 31 (23, 39) |
| 4 – severe (30, 16) | 42 | 9 | 33 (9, 57) |
Source: Adapted from FDA Review
1 Response is defined as a PGA score of 0 (clear) or 1 (almost clear) with a ≥2-grade improvement from baseline.
2 ITT population: all randomized subjects. PGA data obtained from alternative visits (i.e., modified In-clinic visits, phone visits, and virtual visits) are excluded/treated as missing. Missing data were imputed using multiple imputation. Rates displayed are the averages over the 100 imputed datasets.
Abbreviations: CI, confidence interval; ITT, intent-to-treat; PGA, Physician’s Global Assessment; n[Vehicle], number of patients in subgroup for vehicle treatment arm; n[VTAMA], number of patients in subgroup for VTAMA treatment arm
Table 4. PGA Response1 at Week 12 by Age, Sex, Race, Ethnicity, Country, and Baseline PGA Score (ITT2) for Trial DMVT-505-3002
| Subgroup (n[VTAMA], n[Vehicle]) |
VTAMA N=340 % |
Vehicle N=170 % |
Difference % (95% CI) |
| Age, years | |||
| <65 (298, 144) | 39 | 6 | 33 (26, 41) |
| ≥65 (45, 28) | 46 | 10 | 36 (17, 56) |
| Sex | |||
| Male (188, 102) | 42 | 5 | 37 (28, 46) |
| Female (155, 70) | 38 | 8 | 30 (19, 40) |
| Race | |||
| White (300, 138) | 41 | 6 | 35 (28, 42) |
| Black (12, 6) | 27 | 17 | 11 (-29, 50) |
| Asian (25, 21) | 34 | 7 | 28 (4, 51) |
| Other (6, 7) | 33 | 3 | 30 (-11, 72) |
| Ethnicity | |||
| Hispanic (43, 23) | 37 | 6 | 31 (12, 50) |
| Not Hispanic (300, 148) | 41 | 7 | 34 (27, 41) |
| Not reported (0, 1) | - | 0 | - |
| Country | |||
| United States (266, 133) | 39 | 7 | 32 (24, 39) |
| Canada (77, 39) | 45 | 4 | 41 (27, 54) |
| Baseline PGA score | |||
| 2 – mild (28, 15) | 24 | 7 | 17 (-4, 38) |
| 3 – moderate (288, 144) | 43 | 7 | 36 (28, 43) |
| 4 – severe (27, 13) | 31 | 1 | 30 (11, 50) |
Source: Adapted from FDA Review
1 Response is defined as a PGA score of 0 (clear) or 1 (almost clear) with a ≥2-grade improvement from baseline.
2 ITT population: all randomized subjects. PGA data obtained from alternative visits (i.e., modified In-clinic visits, phone visits, and virtual visits) are excluded/treated as missing. Missing data were imputed using multiple imputation. Rates displayed are the averages over the 100 imputed datasets.
Abbreviations: CI, confidence interval; ITT, intent-to-treat; PGA, Physician’s Global Assessment; n[Vehicle], number of patients in subgroup for vehicle treatment arm; n[VTAMA], number of patients in subgroup for VTAMA treatment arm
What are the possible side effects?
The most common side effects of VTAMA include inflamed hair pores (folliculitis); the common cold, sore throat, and sinus infection (nasopharyngitis); local skin irritation (contact dermatitis); headache; itchiness (pruritus); and influenza.
What are the possible side effects (results of trials used to assess safety)?
Table 5 summarizes the adverse reactions that occurred in at least 1% of patients in the VTAMA group and at a higher rate than the placebo group during the 12-week controlled period. Presented is the safety population that includes all patients who received treatment at least once according to the approved dose regimen.
Table 5. Adverse Reactions Occurring in ≥1% of the Subjects in the 12-Week PSOARING 1 and PSOARING 2 Clinical Trials
| Adverse Reaction | VTAMA N=683 n (%) |
Placebo N=342 n (%) |
| Folliculitisa | 140 (20) | 3 (1) |
| Nasopharyngitisb | 73 (11) | 31 (9) |
| Contact dermatitisc | 45 (7) | 2 (1) |
| Headached | 26 (4) | 5 (1) |
| Prurituse | 20 (3) | 2 (1) |
| Influenzaf | 14 (2) | 2 (1) |
Source: VTAMA Prescribing Information
a Folliculitis includes application site folliculitis and folliculitis
b Nasopharyngitis includes nasopharyngitis, nasal congestion, pharyngitis, respiratory tract infection (RTI) viral, rhinorrhea, sinus congestion, upper RTI, and viral upper RTI
c Contact dermatitis includes dermatitis, contact dermatitis, hand dermatitis, and rash
d Headache includes headache, migraine, and tension headache
e Pruritus includes application site pruritus, pruritus, generalized pruritus, and genital pruritus
f Influenza includes influenza and influenza-like illness
Were there any differences in side effects among sex, race and age?
- Sex: Folliculitis and pruritus were more likely to occur in males compared to females. Nasopharyngitis, contact dermatitis, and headache were slightly more common in females.
- Race: Asians were more likely to develop folliculitis and pruritus than Whites or Blacks. However, the majority of patients were White; therefore, no definitive conclusions across races can be drawn.
- Age: Subjects aged 66 and older were more likely to experience contact dermatitis and pruritus than younger subjects.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6. TEAEs by Sex, VTAMA Subjects, Phase 3 Studies 3001 and 3002
| Preferred Term | Males N=401 n (%) |
Females N=282 n (%) |
| Folliculitisa | 101 (25.2) | 42 (14.9) |
| Nasopharyngitisb | 42 (10.5) | 36 (12.8) |
| Contact dermatitisc | 24 (6) | 21 (7.4) |
| Pruritusd | 19 (4.7) | 7 (2.5) |
| Headachee | 14 (3.5) | 12 (4.3) |
Source: Adapted from FDA Review
a Folliculitis includes folliculitis, application site folliculitis, keratosis pilaris
b Nasopharyngitis includes nasopharyngitis, nasal congestion, pharyngitis, respiratory tract infection viral, rhinorrhea, sinusitis, sinus congestion, upper respiratory tract infection, viral sinusitis, viral upper respiratory tract infection
c Contact dermatitis includes dermatitis, dermatitis contact, dermatitis allergic, hand dermatitis, neurodermatitis, rash
d Pruritus includes application site irritation/pain/pruritus, burning sensation, pruritus, genital burning sensation, pruritus generalized, pruritus genital, skin burning sensation, pain of skin
e Headache includes headache, migraine, tension headache
Abbreviations: TEAE, treatment-emergent adverse event
Table 7. Adverse Reactions by Race, VTAMA Subjects, Phase 3 Studies 3001 and 3002
| Preferred Term | White N=586 n (%) |
African-American N=282 n (%) |
Asian N=46 N(%) |
| Folliculitisa | 119 (20.3) | 6 (20) | 14 (30.4 |
| Nasopharyngitisb | 70 (11.9) | 3 (10) | 2 (4.3) |
| Contact dermatitisc | 45 (7.7) | 0 (0) | 0 (0) |
| Pruritusd | 24 (4.1) | 1 (3.3) | 1 (2.2) |
| Headachee | 21 (3.6) | 0(0) | 5 (10.9) |
Source: Adapted from FDA Review
a Folliculitis includes folliculitis, application site folliculitis, keratosis pilaris
b Nasopharyngitis includes nasopharyngitis, nasal congestion, pharyngitis, respiratory tract infection viral, rhinorrhea, sinus congestion, upper respiratory tract infection, viral pharyngitis, viral upper respiratory tract infection
c Contact dermatitis includes dermatitis, dermatitis contact, application site dermatitis, hand dermatitis, neurodermatitis, rash, rash popular, rash pruritic
d Pruritus includes application site irritation/pain/pruritus, burning sensation, pruritus, skin irritation
e Headache includes headache, migraine, migraine with aura, tension headache
Abbreviations: LTE, long-term extension
Table 8. Adverse Reactions by Age Group, LTE Study 3003
| Preferred Term | 18 to 65 Years N=685 n (%) |
≥66 Years N=78 n (%) |
| Folliculitisa | 174 (25.4) | 11 (14.1) |
| Nasopharyngitisb | 68 (9.9) | 3 (3.8) |
| Contact dermatitisc | 52 (7.6) | 6 (7.7) |
| Pruritusd | 21 (3.1) | 3 (3.8) |
| Headachee | 15 (2.2) | 1 (1.3) |
Source: Adapted from FDA Review
a Folliculitis includes folliculitis, application site folliculitis, keratosis pilaris
b Nasopharyngitis includes nasopharyngitis, nasal congestion, pharyngitis, respiratory tract infection viral, rhinorrhea, sinus congestion, upper respiratory tract infection, viral pharyngitis, viral upper respiratory tract infection
c Contact dermatitis includes dermatitis, dermatitis contact, application site dermatitis, hand dermatitis, neurodermatitis, rash, rash popular, rash pruritic
d Pruritus includes application site irritation/pain/pruritus, burning sensation, pruritus, skin irritation
e Headache includes headache, migraine, migraine with aura, tension headache
Abbreviations: LTE, long-term extension
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.