Axonics Sacral Neuromodulation (SNM) System for Urinary Control – P180046
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Axonics Sacral Neuromodulation System
PMA Applicant: Axonics Modulation Technologies, Inc.
Address: 26 Technology Drive, Irvine, CA 92618
Approval Date: November 13, 2019
Approval Letter: Approval Order
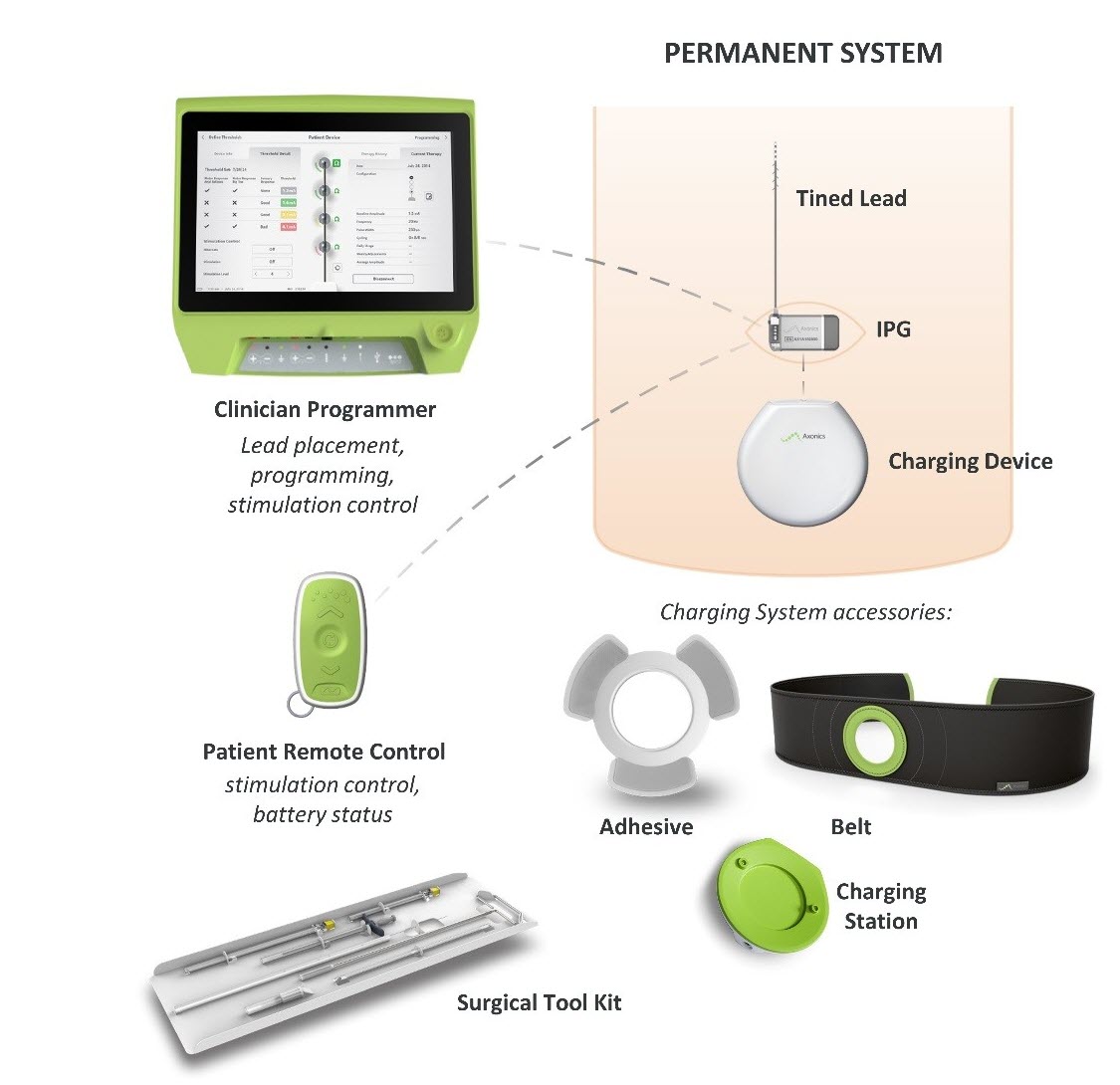
What is it? The Axonics Sacral Neuromodulation (SNM) System is a sacral nerve stimulation (SNS) system that is intended to treat urinary retention and the symptoms of overactive bladder. It uses an implanted stimulator to deliver electrical pulses through a lead wire to electrodes located near the sacral nerve.
How does it work? An implantable pulse generator (IPG) is surgically placed below the skin in the upper buttock area and attached to a lead wire that passes between the hip bones of the pelvis and ends at the sacral nerve. The IPG sends electrical pulses through the lead wire to the sacral nerve that travels from the spinal cord to the bladder to help improve urinary control.
When is it used? The Axonics SNM System for urinary control is used for the treatment of urinary retention and the symptoms of overactive bladder in patients who have failed or could not tolerate other treatments, such as lifestyle changes, pelvic floor exercises, or medication. If left untreated, urinary retention and overactive bladder can reduce a patient’s quality of life and can result in significant challenges in maintaining activities of daily living.
What will it accomplish? The results from a clinical study of the Axonics SNM System combined with additional clinical data compiled from a systematic review of literature available for a similar SNM device show that there is benefit for most patients with urinary retention and the symptoms of overactive bladder who have failed or could not tolerate other treatments. In the Axonics clinical study of patients with urge urinary incontinence, 90% (116/129) of patients with the implanted SNM System achieved at least a 50% reduction in the number of urgency leaks at 6 months compared to the number of urgency leaks without the SNM System. In a longer-term study (van Kerrebroeck, 2007), 152 patients implanted with a similar SNM device were followed for 5 years. At 5 years, 58% (56/96) of patients with urge urinary incontinence had at least a 50% improvement (measured in terms of the number of leaks per day). Additionally after 5 years, 40% (10/25) of patients with urgency-frequency had at least a 50% improvement (measured in terms of the number of voids per day), and 58% (18/31) of patients with urinary retention had at least a 50% improvement (measured in terms of the number of catheterizations per day). Given the similarities in design, technology, performance, indications for use, output characteristics and the patient population it is meant to treat, FDA believes that the Axonics SNM System will have similar performance to the commercially available SNM system evaluated in the published studies.
When should it not be used? The Axonics SNM System should not be used in:
- Patients who have not demonstrated an appropriate response to test stimulation; or
- Patients who are unable to operate the Axonics SNM System
Additional information (including warnings, precautions, and adverse events):