Bulkamid Urethral Bulking System - P170023
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Bulkamid Urethral Bulking System
PMA Applicant: Contura International A/S
Address: Sydmarken 23, 2860 Soborg, Denmark
Approval Date: January 28, 2020
Approval Letter: Approval Order
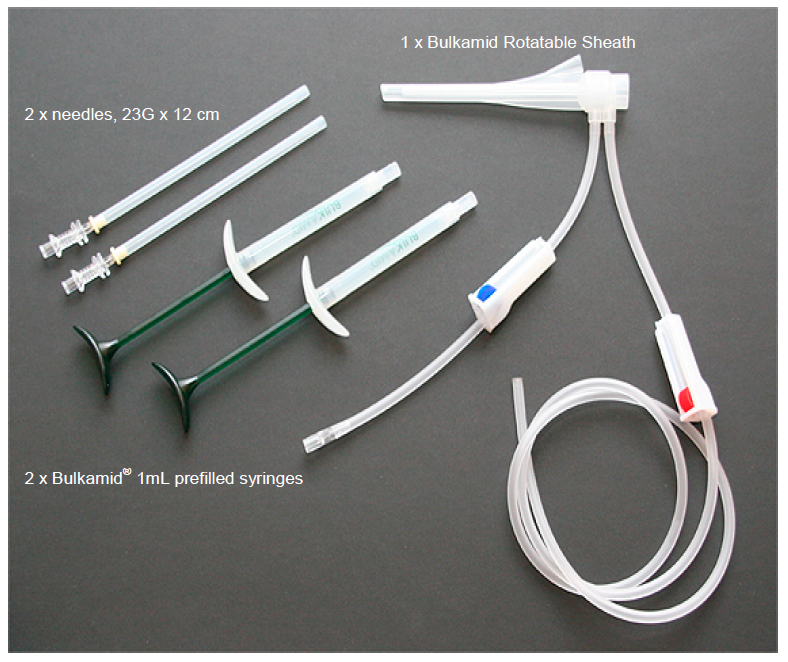
What is it? Bulkamid is a thick, permanent gel used to treat women who have stress urinary incontinence due to weak pelvic floor (urethral sphincter) muscles. The Bulkamid Urethral Bulking System is a single-use kit that consists of sterile syringes filled with the Bulkamid gel, a rotatable sheath, and injection needles.
How does it work? A doctor injects Bulkamid into the wall of the urethra near the bladder. After Bulkamid is injected into the body, it adds volume to the wall of the urethra to help provide support of the urethra and better control of urine flow.
When is it used? One cause of stress urinary incontinence is weak urethral sphincter muscles that help open and close the tube from the bladder that drains urine from the body (urethra). Bulkamid may be used for adult women with stress urinary incontinence who may experience uncontrolled urination during exercise or other body movements such as sneezing and coughing.
What will it accomplish? In a clinical study, 47% (107/228) of women had significant improvement in their incontinence 1 year after receiving Bulkamid. These women had at least a 50% reduction in both the amount of urine leaked, and the number of times they leaked urine, in a 24-hour period. About three-quarters of patients (176/228) needed to have the Bulkamid injected again to achieve satisfactory results.
When should it not be used? The device should not be used in women who have signs of a urinary tract infection.
Additional information (including warnings, precautions, and adverse events):