The cobas HPV for use on the cobas 6800/8800 Systems – P190028
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
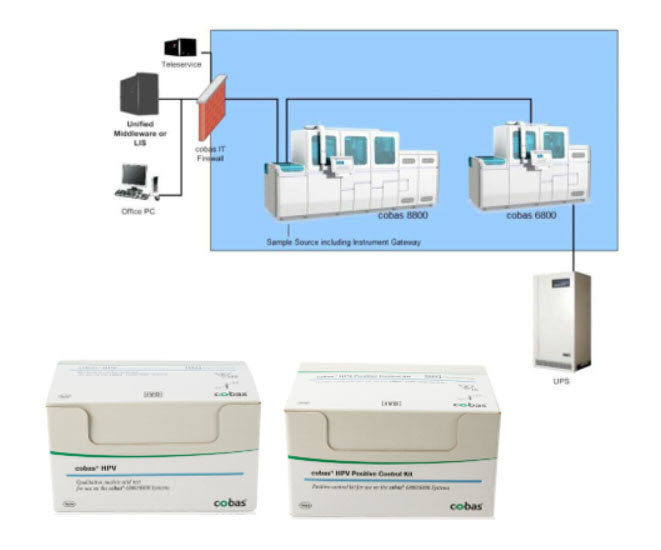
Product Name: cobas HPV for use on the cobas 6800/8800 Systems
Applicant: Roche Molecular Systems, Inc.
Address: 4300 Hacienda Drive, Pleasanton, CA 94588
Approval Date: April 3, 2020
Approval Letter: Approval Order
What is it?
The cobas HPV for use on the cobas 6800/8800 Systems (cobas HPV) is a laboratory test designed to detect human papillomavirus (HPV) in cervical samples.
The cobas HPV consists of the cobas 6800/8800 Systems, cobas HPV analysis software, and chemical substances (reagents) that are used to prepare the cervical samples and for chemical analysis.The test is programmed only for use on the cobas 6800 or 8800 System. The cobas 6800/8800 software processes the data and assigns positive, negative or invalid test results which can be reviewed directly on the system screen, exported, or printed as a report.
How does it work?
A doctor collects a cervical sample using a specific brush or spatula and solution (not included). In the laboratory, the test uses DNA in a cervical sample to identify HPV16 and HPV18 types while also detecting 12 other high risk (HR) types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).
When is it used?
The cobas HPV is indicated for use for routine cervical cancer screening according to professional medical guidelines. This includes testing patients with abnormal cervical cells, co-testing (adjunctive screen) with tests that use cells to diagnose or screen for cancer, and screening patients older than 25 years old for cervical cancer.
What will it accomplish?
In a clincial study, the cobas HPV was shown to be safe and effective to use for routine cervical cancer screening according to professional medical guidelines.
When should it not be used?
There are no known reasons not to use the test.
Additional information (including warnings, precautions, and adverse events):