Exablate Model 4000 Type 1.0 and 1.1 System (“Exablate Neuro”) – P150038/S014
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Exablate Model 4000 Type 1.0 and 1.1 System (“Exablate Neuro”)
PMA Applicant: INSIGHTEC, Inc.
Address: 4851 LBJ Freeway, Suite 400 Dallas, Texas 75244
Approval Date: 10/29/2021
Approval Letter: Approval Order
What is it?
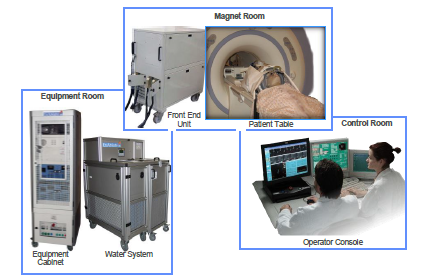
The Exablate Model 4000 Type 1.0 and 1.1 System (Exablate Neuro) is a magnetic resonance image-guided focused ultrasound (MRgFUS) system that treats tremors that cannot be controlled using medication alone in essential tremor and tremor-dominant Parkinson’s disease patients by destroying areas of brain tissue.
This approval expands the indications for use of the Exablate Neuro to include use of this device in the unilateral pallidotomy of medication-resistant Parkinson’s disease patients with moderate to severe motor complications as a supplement to medication treatment.
How does it work?
The Exablate Neuro combines two technologies: magnetic resonance imaging (MRI) to identify the area in the brain that is thought to be responsible for the movement disorder symptoms and a focused ultrasound beam to heat and destroy the tissue.
The ultrasound treatment is applied in incremental increases in energy called sonications. After each sonication, the patient and doctor evaluate the reduction in tremor until a satisfactory result is achieved. Patients are awake and responsive during the entire treatment.
When is it used?
The Exablate Neuro is indicated for use in the unilateral pallidotomy of patients with advanced, idiopathic Parkinson’s disease with medication-refractory moderate to severe motor complications as an adjunct to Parkinson’s disease medication treatment. The designated area in the brain responsible for the movement disorder symptoms [globus pallidus (GPi)] must be identified and accessible for targeted thermal ablation by the Exablate device. Patients must be at least age 30.
The Exablate Neuro is also indicated for use in the unilateral thalamotomy (ventralis intermedius) of tremor-dominant Parkinson’s disease with medication-refractory tremor. Patients must also be at least age 30.
In addition, the Exablate Neuro is indicated for use in the unilateral thalamotomy treatment of idiopathic essential tremor patients with medication-refractory tremor. The designated area in the brain responsible for the movement disorder symptoms (ventralis intermedius) must be identified and accessible for targeted thermal ablation by the Exablate device. Patients must be at least 22 years old.
What will it accomplish?
The device may reduce moderate to severe medication-refractory motor complications in patients when used as a supplement to Parkinson’s disease medication treatment.
Data supporting the approval of this device system included a prospective, two-arm (treatment and sham control groups), randomized (3:1), multi-center clinical trial of 94 patients (69 patients in the Exablate Neuro group, 25 patients in the sham control group).
The trial showed that the Exablate Neuro group demonstrated a 68.6% responder improvement rate, while the sham group demonstrated 33.3% improvement by 3-months post-procedure.
Outcomes were measured using the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III (OFF medication motor exam on the treated side) and the Unified Dyskinesia Rating Scale (UDysRS) Objective Impairment (ON medication) at 3-months post-procedure.
When should it not be used?
The Exablate Neuro should not be used in:
- Patients who should not have an MRI scan. For instance, if they have non-MRI compatible implanted metallic devices including cardiac pacemakers, size limitations, or allergies to MRI contrast agent.
- Patients who are pregnant.
- Patients with advanced kidney disease or on dialysis.
- Patients with unstable heart status or severe high blood pressure.
- Patients showing any behavior(s) consistent with drinking alcohol or substance abuse.
- Patients with history of abnormal bleeding, hemorrhage, or a bleeding disorder where the blood is unable to form blood clots.
- Patients receiving drugs that prevent blood clots or drugs known to increase risk of hemorrhage within one month of focused ultrasound procedure.
- Patients with cerebrovascular disease.
- Patients with brain tumors.
- Patients who are not able or unwilling to tolerate the required prolonged stationary position during treatment. The average treatment time (the time from the first scan to allocate transducer position and ending with the last energy delivery) is 1:56 ± 0.41 hours (hrs) (min: 0.48 hrs, max: 5:54 hrs).
- Patients who have an overall skull density ratio of 0.45 (± 0.05) or less as calculated from the screening computed tomography (CT).
- Parkinson’s disease patients with unstable psychiatric disease, uncontrolled depressive symptoms, psychosis, delusions, hallucinations, or suicidal ideation.