HeartStart FRx Defibrillator (861304), Primary Battery (Model M5070A), Aviation FRx Battery (989803139301), SMART Pads II (Model 989803139261), and Infant/Child Key (Model 989803139311) – P180028
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
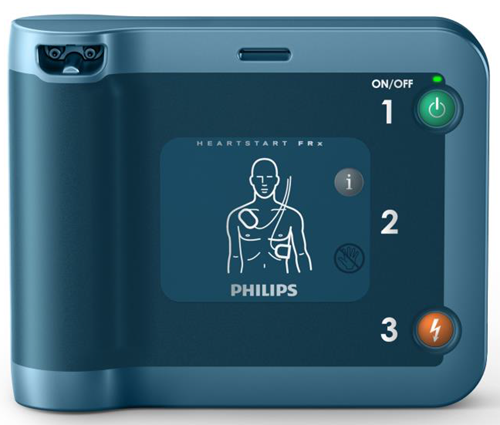
Product Name: HeartStart FRx Defibrillator
PMA Applicant: Philips Medical Systems
Address: 22100 Bothell Everett Hwy, Bothell, WA 98021
Approval Date: May 11, 2020
Approval Letter: Approval Order
What is it? The Philips HeartStart FRx Defibrillator (Model 861304) is a battery-powered automated external defibrillator (AED). The FRx AED is indicated for use on potential victims of sudden loss of heart function (sudden cardiac arrest or SCA).
How does it work? The HeartStart FRx uses two (2) defibrillation electrodes (sensors), placed on the patient’s chest, to get a patient’s electrical activity of the heart (electrocardiogram or ECG). If this device detects an abnormal heartbeat, it may advise the user that a “shock” from the defibrillator to restore the heart’s normal pumping is appropriate. If an electric shock is required, the device will deliver a controlled shock to the patient through the multifunction defibrillator electrodes to restore the patient’s heart to a normal rhythm. The user interface will provide voice and text/icon prompts to guide the user through the rescue process, including shock delivery and/or cardiopulmonary resuscitation (CPR).
When is it used? The HeartStart FRx Model 861304 is indicated for use by trained responders to treat abnormal heart rhythm (ventricular fibrillation or VF), the most common cause of sudden loss of heart function (sudden cardiac arrest or SCA), and pulseless rapid heartbeat (ventricular tachycardias or VTs) on adults over 55 pounds (25 kg). The Model 861304 is also indicated for infants and children under 55 pounds (25 kg) or 0-8 years old when used with the optional Infant/Child Key (Model 989803139311). If the Infant/Child Key is not available, or you are uncertain of the child’s age or weight, proceed using adult treatment without the infant/child key.
What will it accomplish? The combination of CPR and early defibrillation is highly effective in saving patients lives when used in the first few minutes following collabpse from sudden loss of heart function. For every minute that passes between collapse and defibrillation, survival rates from SCA decrease by 7-10%. Use of the HeartStart FRx device can reduce the time in cardiac arrest and increase the chance of patient survival.
When should it not be used? The HeartStart FRx Defibrillator should not be used when a patient is conscious, breathing, or has a detectable pulse or other signs of circulation.
Additional information (including warnings, precautions, and adverse events):